Sclerotherapy for a giant lymphatic malformation in the neck: a case report
Introduction
Lymphatic malformation, also known as lymphangioma, is caused by the abnormal development of the local area of the lymphatic system (1). Although a few cases have been reported in adults, about 50% cases occur at birth and 90% cases occur in children aged <2 years; 75% lymphatic malformations in these cases occur in the head and neck. The incidence of this disease is approximately 8–180 per 100,000 people, and there is no significant relationship with gender and ethnicity (2-4). Lymphatic malformations are divided into the following 3 types based on the size: macrocystic, microcystic, and mixed type (5); mixed type lymphatic malformations are more commonly observed in clinical practice. Although a benign lesion, it rarely regresses naturally (6). Traditionally, common treatments for lymphatic malformations include surgical resection, sclerotherapy, and laser treatment (7). Thus far, no consensus has been reached regarding an optimal therapeutic schedule, but an appropriate therapeutic schedule should be made considering the volume, location, and type of lesion; location of the malformation in other important areas; and the presence of dysfunctions.
Case presentation
A boy, born with a large lump in the left side of the neck, presented to our hospital 5 days later on January 29, 2018 (Figure 1). Physical examination revealed that the left neck mass was raised above the surrounding skin with an unclear boundary by naked eye. There was no pain upon pressure, and the skin temperature was normal. Pulsation was not obvious, and there was no rupture or infection. The child experienced difficulty in breathing and made a snoring sound while sleeping. Ultrasound examination revealed a cystic mass under the skin and multiple sections without obvious blood flow signals. The lymphatic malformations were approximately 8.0 cm × 8.0 cm × 3.6 cm in size (Figure 2). The child weighed 3 kg and was too young for surgery and the polycystic lesion located in the neck had the potential to affect breathing; hence, we considered sclerotherapy as a treatment option. The injectable drugs included lauromacrogol foam and triamcinolone acetonide. Lauromacrogol solution (1%) and air were quickly mixed in a 1:4 ratio to make foam using the Tessari method (8). Triamcinolone acetonide was diluted to 13.3 mg/mL with 2 mL of 0.9% normal saline. A small amount of lauromacrogol foam and triamcinolone acetonide was injected in multiple areas using the double-needle method. Two 4.5# scalp needles were placed above and below a certain area to inject each sclerosing agent. The cystic fluid and a small amount of sclerosing agent was taken out. A faint yellow clear liquid was extracted from multiple points in the cyst. Cytological examination revealed the presence of many mature lymphocytes in the liquids; no pathological cells were found. Another two rounds of treatment, each treatments with an interval of 3 weeks, did not have a satisfactory effect and the sac fluid gradually became dark red.
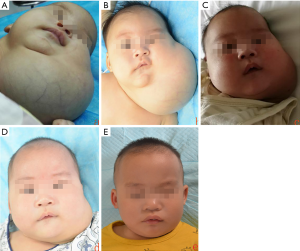
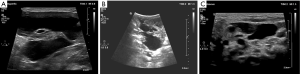
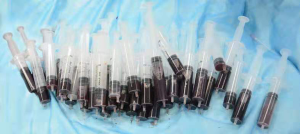
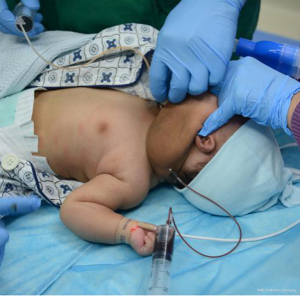
By April 8, 2018, when the child was 2.5 months old, the size of the lesion has increased to 11.5 cm × 12.0 cm × 7.7 cm (Figure 1B) and the child’s body weight was 4.5 kg. Pingyangmycin was added at the fourth treatment. Pingyangmycin was diluted with 4 mL of 0.9% normal saline and 2 mL multipoint injections were given. After a small dose of the sclerosing agent into cavity, it was washed with a small amount of 0.9% normal saline and was inserted from above and removed from below to reduce excess drug in the capsule and reduce side effects. For this procedure, 330 mL of cyst fluid was removed (Figure 3). Figure 4 shows the double-needle injection method used during the operation. After the operation, the child was transferred to the pediatric intensive medicine department at our hospital. The child’s postoperative condition remained stable and no special treatment was given. The child was discharged after 3 days post-operation. During the 1-month treatment interval for pingyangmycin (9), and the child was closely followed up. During the follow up, the condition of the child was stable and the size of the lesion had significantly reduced. On April 22, 2018, the child was re-examined (Figure 1C) and the previous treatment procedure was repeated. After the operation, the child was again transferred to the pediatric intensive medicine department for 1 day. The child’s postoperative condition was stable and was discharged without any special treatment. On May 29, 2018, the child was re-examined (Figure 1D), and the ultrasound examination showed a lesion of about 7.5 cm × 9.0 cm × 5.12 cm in size (Figure 2B). The treatment plan was continued as described above.
Subsequently, the child was closely followed up once a month, and the size of the lesion was gradually reducing. On October 15, 2018, a reexamination ultrasound revealed that size of the lesion was reduced to approximately 6.0 cm × 2.3 cm × 6.1 cm, and the child’s condition was stable. Therefore, we decided not to intervene any further. On December 18, 2018, the child’s parents complained of a sudden increase in the size of the lesion within 1 week and visited our hospital for treatment. Ultrasound results showed lymphatic malformations combined with hemorrhage. We continued the treatment that was previously effective. During the operation, 45 mL of dark red liquid was drained and the previous treatment plan was continued. On March 20, 2019, when the patient was 1 year and 2 months old, the lesion was stable (Figure 1E). Ultrasound results revealed that the size of the lesion was further reduced to approximately 5.6 cm × 3.4 cm × 5.3 cm (Figure 2C); however, the child was continued to be followed up.
Discussion
Head and neck lymphatic malformations account for 75% of all lymphatic malformations, and this may be due to the abundance of the lymphatic system in the head and neck (7). Under normal circumstances, lymphatic malformations grow alongside the growth of the child, but there are two worrying complications, infection and intralesional bleeding (1). In our case, the child also suffered from intralesional bleeding during the therapeutic process. Lymphatic malformations are regarded as benign lesions, but more attention should be paid to these in clinical practice (10). Even if the malformation is controlled, it still needs daily care and attention.
Traditionally, surgical resection was preferred treatment option (11); however, many researchers currently believe that sclerotherapy is a good alternative to surgical treatment (12). There are many types of sclerosing agents. Yura et al. were the first to use bleomycin injection for the treatment of lymphatic malformations in 1977 (13). Recently, treatment with pingyangmycin has become popular in China. Pingyangmycin, also known as bleomycin A5, has shown good results (14). Furthermore, triamcinolone acetonide can also enhance the efficacy of pingyangmycin (15). Wang et al. showed that lauromacrogol foam is effective for treating head and neck lymphatic malformations (8). Moreover, the treatment of cervical lymphatic malformations requires a multidisciplinary team, involving the surgery, anesthesia, pediatric, and imaging departments. Even if the treatment is nearly complete, cervical lymphatic malformations cannot be taken lightly; participation of a multidisciplinary team provides safer and more effective treatment outcomes.
Acknowledgments
We would like to thank Dr. Qi Hengtao from the Ultrasound Diagnosis Laboratory of Shandong Medical Imaging Research Institute for the imaging data. We are grateful for the surgical and nursing support provided by the Department of Anesthesiology and Pediatric Intensive Care Unit of Shandong Provincial Hospital affiliated to Shandong University.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://fomm.amegroups.org/article/view/10.21037/fomm.2019.09.01/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013).Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mulliken JB, Burrows PE, Fishman SJ. Mulliken and Young's Vascular Anomalies, Second edition. Oxford University Press, New York, 2013;842-6.
- Francavilla ML, White CL, Oliveri B, et al. Intraabdominal Lymphatic Malformations: Pearls and Pitfalls of Diagnosis and Differential Diagnoses in Pediatric Patients. AJR Am J Roentgenol 2017;208:637-49. [Crossref] [PubMed]
- Division of Vascular Anomalies, Chinese Society of Oral and Maxillofacial Surgery, Chinese Stomatological Association. The protocol of treatment guideline of oral and maxillofacial lymphatic malformations. China J Oral Maxillofac Surg 2010;8:386-90.
- Kennedy TL, Whitaker M, Pellitteri P, et al. Cystic hygroma/lymphangioma: a rational approach to management. Laryngoscope 2001;111:1929-37. [Crossref] [PubMed]
- ISSVA classification for vascular anomalies [EB/OL]. [published May 2018, accessed July 2019]. Available online: http://www.issva.org/classification
- Van Aalst JA, Bhuller A, Sadove AM. Pediatric vascular lesions. J Craniofac Surg 2003;14:566-83. [Crossref] [PubMed]
- Zhang Z, Zhao Y, Zheng J, et al. Hemangioma and Vascular Malformations of the Head and Neck. Shanghai World Publishing Corporation, Shanghai, 2007; 65, 116, 126.
- Wang L, Liu F, Huang S. Percutaneous Lauromacrogol Foam Sclerotherapy for the Treatment of Acute Airway Compression Caused by Lymphatic Malformations in Infants. Biomed Res Int 2018;2018:3878960. [PubMed]
- The Instruction Manual of Bleomycin A5 Hydrochloride for Injection. 2015. Jilin Aodong Pharmaceutical Group CO., LTD. Available online: http://www.jlaod.com/#/page?id=4c8323ee59504b34a86344c2d3092866
- Zhang W, Luo Q, Zhang L, et al. The therapeutic effect of triamcinolone acetonide with Pingyangmycin on lymphatic malformations in oral and maxillofacial regions. Zhonghua Zheng Xing Wai Ke Za Zhi 2010;26:262-5. [PubMed]
- Orvidas LJ, Kasperbauer JL. Pediatric lymphangiomas of the head and neck. Ann Otol Rhinol Laryngol 2000;109:411-21. [Crossref] [PubMed]
- Madsen HJ, Annam A, Harned R, et al. Symptom Resolution and Volumetric Reduction of Abdominal Lymphatic Malformations With Sclerotherapy. J Surg Res 2019;233:256-61. [Crossref] [PubMed]
- Yura J, Hashimoto T, Tsuruga N, et al. Bleomycin treatment for cystic hygroma in children. Nihon Geka Hokan 1977;46:607-14. [PubMed]
- Zhou Q, Zheng J, Mai H, et al. Treatment guidelines of lymphatic malformations of the head and neck. Oral Oncol 2011;47:1105-9. [Crossref] [PubMed]
- Luo QF, Gan YH. Pingyangmycin with triamcinolone acetonide effective for treatment of lymphatic malformations in the oral and maxillofacial region. J Craniomaxillofac Surg 2013;41:345-9. [Crossref] [PubMed]
Cite this article as: Li Z, Lv R, Xu G, Huo R. Sclerotherapy for a giant lymphatic malformation in the neck: a case report. Front Oral Maxillofac Med 2019;1:7.