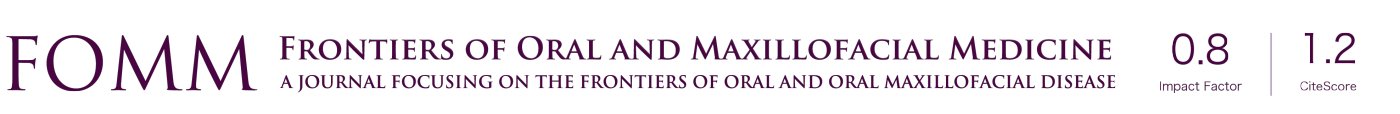The role of skeletal surgical treatment in the management of obstructive sleep apnea: an overview
Introduction
Obstructive sleep apnea (OSA) is an underdiagnosed sleep-related breathing disorder. Not only does it lack public awareness, but even clinicians and dentists may have had underestimated the seriousness of the condition to keep us alert in actively diagnosing them. In Hong Kong, the prevalence were 4.1% (1) and 2.1% (2) among middle-aged men and women respectively, which is similar worldwide. Male gender, old age, obesity, increased neck circumference and snoring are typical risk factors associated with OSA (3,4). OSA has been shown to be associated with multiple cardiovascular diseases (CVD) including hypertension, myocardial infarction, angina, heart failure, and stroke, as well as other metabolic diseases such as diabetes (5,6). Epilepsy was also found to be related to OSA, possibly resulting from hypoxaemia-mediated brain damage (7). In addition, research showed associations between OSA and increased severity of Parkinson’s disease-associated cognitive and motor dysfunctions (8). Apart from medically related comorbidities, OSA patients suffer from excessive daytime sleepiness which adversely affect their normal functioning in everyday life like diminished work performance and difficulty staying awake while driving (9-11). Appropriate screening, diagnosis and treatment are important for OSA patients and to prevent these effects.
Management of OSA can be categorized into non-surgical and surgical modalities. Non-surgical methods are usually advocated before surgical treatment. They include lifestyle changes, continuous positive airway pressure (CPAP), oral appliances, behavioral measures, positional therapy, nasal resistors, and myofunctional therapy. For many years, CPAP has been and continues to be the golden standard of treatment for OSA. It was shown to be effective in managing moderate to severe OSA (7). The cure rate of patients with mild to moderate OSA was found to be 73.2% in CPAP users (12). However, the non-adherence rate is as high as 34.1% (13), and there are numerous accompanying side effects. Nasal congestion happens in 65% of patients using CPAP. Other side effects include dry nose or throat. Mouth leak can also occur and affects the efficacy or pressure delivery to upper airway (14). CPAP is bulky and noisy, causing intolerance to patients and bed partners. It provides only a temporary cure to the patient while they are putting on the machine. Patients’ refractory or intolerant to non-surgical approaches may then consider surgical approaches. Obese patients may consider receiving bariatric surgery first, then re-evaluate the necessity for other surgeries. Upper airway operations are performed according to the anatomic locations of obstruction in the nasal cavity, nasopharyngeal, oropharyngeal and hypopharyngeal regions, and the soft and/or hard tissues involved.
With the complexity in the management of OSA patients, multi-disciplinary treatment provides the best outcome. Soft tissue surgeries can involve removal of excessive tissues or re-approximating tissues at tonsils, pharynx, uvula, and/or tongue base, thus expanding the airway and reduces obstruction. Traditional thinking believes major skeletal surgeries were indicated only if soft tissue surgeries failed or relapse occurred. Recently, there has been an increasing trend to adopt bony surgeries as first-line in suitable cases. Although skeletal surgeries are relatively more extensive and potentially possess greater risk, the upper airway architecture is reconstructed to a new position, which supposedly may bring a more long-lasting effect, with no extra prostheses or machines needed to maintain the outcome. As an important treatment modality of moderate-to-severe OSA conditions, it is important for clinicians to understand the principles, outcome, and risks of the most common skeletal procedures performed. This article aims at reviewing common skeletal surgeries as the treatment of OSA.
Diagnosis, treatment objectives and surgical planning
OSA is commonly undiagnosed and clinicians should screen routinely for patients who have suspicious OSA risk factors or anatomical presentations. The screenings identify individuals with excessive daytime sleepiness or signs of obstructive features. Common screening tools include the Epworth Sleepiness Scale (ESS) (15), the Berlin questionnaire (BQ) (16), the “STOP” questionnaire (17), and “STOP-BANG” questionnaire (SBQ) (18). Although SBQ was found to be more accurate in detecting OSA (19), ESS is more commonly used and many papers evaluated the treatment success with ESS. ESS is also a simple questionnaire for easy assessment, which avoids much clinical time to come up with a validated screening of potential OSA cases.
Diagnosis of OSA
After initial screening for OSA, a polysomnography (PSG) confirms the diagnosis and grades the severity with the apnea-hypopnea index (AHI). It measures the number of apnea and hypopnea events per hour of sleep. Apnea is defined as the absence of airflow for at least 10 s. Hypopnea means at least 30% decrease in airflow for at least 10 s with accompanying reduction in oxygen saturation. Respiratory disturbance index (RDI) has a similar definition, but it also takes into account the respiratory effort-related arousal (RERAs). An AHI <5 per hour indicates no or minimal OSA; an AHI of ≥5 but <15 shows a mild OSA; ≥15 but <30 for moderate; and ≥30 for severe OSA. Other data available in a PSG report includes lowest oxygen saturation (LSAT), eye movements, leg movements and brain waves, which all complement a detailed sleep study on the parameters related to OSA and indicate different perspectives of the condition.
Treatment objectives
The objective of sleep surgeries lies in minimizing obstruction of upper airway during sleep. Most studies regard treatment success as AHI ≤20 and/or 50% reduction, and treatment cure as AHI <5. Treatment success and cure are important parameters, especially in the era of evidence-based medicine, in assessing treatment outcomes of various surgical modalities in daily clinical settings and clinical trials.
Following the diagnosis of OSA, drug induced sleep endoscopy (DISE) is useful to deduce the exact site(s) of obstruction. DISE mimics the patient’s sleep and the endoscopy may identify the area of obstruction through direct vision and video recording (Figure 1). The Muller’s Maneuver (MM) could be utilized if DISE cannot be performed. An endoscope investigation is performed to identify the area of collapse by asking the patient to inhale with mouth closed and nose plugged. It has been shown that DISE and MM do not consistently correlate with each other in investigating levels of collapse (20,21). DISE was recommended to analyze the different levels of obstruction in anteroposterior, lateral, and concentric dimensions (22,23). The areas of obstruction can be divided into nasal cavity, nasopharynx, oropharynx, and hypopharynx. Another classification uses the acronym VOTE to represent velum, oropharynx, tongue base, and epiglottis. Subsequently, corresponding soft tissue and skeletal surgeries can be planned according to the results at targeted anatomical locations. Simultaneous multilevel surgeries are often warranted given that OSA is rarely contributed by one single site of upper airway blockage.
Skeletal surgeries
Surgical treatment is indicated when non-surgical / medical treatment is not useful or not well-tolerated. Soft tissue surgeries, like uvulopalatoplasties (UPPP) or tongue base reduction, aim to remove or realign redundant soft tissue and to re-create a patent airway during sleep. Hard tissue/skeletal surgeries are performed by osteotomies of the jaw bone and to position the bony base with the attaching soft tissue to a new planned location, thus enlarging the upper airway altogether. This review focuses in discussing the four common skeletal surgeries, namely surgical assisted rapid maxillary expansion, maxillomandibular advancement (MMA), genial tubercle advancement (GTA), and distraction osteogenesis (DO).
Surgically assisted rapid maxillary expansion
Rapid maxillary expansion (RME) is a common treatment option for paediatric patients with OSA when tonsils enlargement as a cause has been ruled out. Before growth cessation, the midpalatal suture can be opened by orthodontic appliance to expand the maxilla. For older children or adults with transverse maxillary deficiency and deep palatal vault after fusion of the palatal suture, surgically assisted rapid maxillary expansion (SARME), also known as surgically assisted rapid palatal expansion (SARPE), is needed. The idea of combination of orthodontics and surgery for maxillary expansion was first proposed in 1938 (24) which was then modified. SARPE involves a vertical osteotomy in the midline and the same cuts as in a Le Fort I osteotomies without down-fracturing the maxilla. A maxillary expander (Figure 2) is inserted pre-operatively and tested during surgery to ensure the mid-palatal suture is released. The expander can be tooth- or tissue-tooth-borne or bone-borne. Following a few days after surgery, the expander can be activated following a stabilization period. A DOME technique (Distraction Osteogenesis Maxillary Expansion), in which a custom-made expander supported by 4 to 6 bone-borne mini-implants is placed, followed by a similar surgery as SARPE is proposed for a similar rationale (25). The expanded maxilla allows a sequential expansion of the nasal cavity as well as the nasal pharynx in a transverse dimension, thus enlarging the airway and in particular the airflow of the nasal cavity to improve OSA.
As a technique that has stood the test of time, RME was shown to be effective in decreasing the AHI in children (26). Regarding SARPE, Vilani et al. has found a significant increase in the mean inter-canine width of 5.62 mm. However, a statistically significant relapse of 1.50 mm was also noted (27). Vinha et al. showed the mean ESS score dropped from 12.5 to 7.2, and a 56.24% reduction of mean AHI was found (from 33.23 to 14.54) (28), which demonstrated a treatment success. With respect to the relatively new technique DOME, it was found to bring a significant decrease in the mean ESS score (from 10.48 to 6.69) and AHI (from 17.65 to 8.17) (29).
For the potential complications, the reported rate of adverse events related to SARME is 21.97%, with minor complications being 78.87%, including epitaxis (2.47%), pain (2.00%), periodontal bone loss, tooth darkening or mobility, wound dehiscence, numbness, infection, headache, etc. Major complications may require further surgeries to rectify the issues. Among them, 84.4% was incorrect expansion and asymmetry. Other less common major complications include tooth resorption, loss of tooth, severe bleeding, palatal fistula, tissue necrosis, and risk of death (30). The overall risk of SARME is low. DOME has a similar risk when compared to conventional SARME. External resorption and chronic infection of central incisors, non-union of maxilla, lack of bone fill in palatal gap were reported (31).
MMA
Mandibular advancement was mentioned in 1978 by J.H. Priest as a treatment of OSA (32). MMA was later introduced in 1986 by Riley et al. (33). The concept of double jaw advancement aims to three dimensionally enlarge the whole airway by moving the bimaxillary complex and the related muscle attachment forward by a significant amount. The muscle attachments of the mandible and the hyoid bone, as well as the muscles in the soft palate would be altogether advanced and tightened to avoid concentric collapse of these structures. Riley and Powell suggested a 2-phase treatment, that involved a combination of soft and hard tissue surgeries (34). Phase 1 involved an uvulopalatopharyngoplasty (UPPP) and/or mandibular osteotomy with genioglossus advancement-hyoid myotomy and suspension. PSG would then be conducted after 6 months and if the result was not satisfactory, a phase 2 of MMA would be conducted, which involved a standard Le Fort I osteotomy and bilateral sagittal split osteotomies (BSSO). Liu et al. also suggested with a detail protocol the technique from preoperative planning, anaesthetic approach, to surgical procedures in their center (35). Compared to the usual orthognathic patients, it was suggested that OSA patients have longer upper airway length, less cancellous bone from aging, higher association with cardiovascular diseases, and greater muscle pull from large advancements (35). To overcome these problems accordingly, it was suggested to use a microlaryngoscopy tube (MLT) and to avoid prolonged operation under overly low mean arterial pressure. Counter-clockwise rotation to improve the airway could also be utilized to open up the airway at the tongue base region, in particular in cases when maxillary advancement might not be achievable to a large extent (36). A systematic review and meta-analysis showed MMA with counter-clockwise rotation significantly increases the volumes and areas of the upper airway spaces (37). Simultaneous septoplasty, widening of nasal floor and piriform rim, inferior turbinectomies, nasal polypectomy, genioglossal advancement should be planned accordingly to address multilevel areas of obstruction. However, the facial profile and soft tissue thickness are different among races and individuals. Concerns have been raised that non-segmental Le Fort I osteotomy and BSSO may be detrimental to facial aesthetics to cause excessive protrusion, especially in Asian patients with class I occlusion. A modified MMA approach with segmental anterior subapical osteotomies (ASO) have been proposed dating back from 2003 by Goh et al. (38-40). Maxillary ASO can allow setback of maxillary incisal point while maintaining the AP dimension or even protracting the posterior maxilla (Figure 3). Mandibular setback ASO prevents worsening of labiomental fold from large genioglossal advancement and provides a greater degree of advancement from the ramus surgeries. The inverted-L ramus osteotomy was compared with SSO for MMA and was found to be both effective (41).
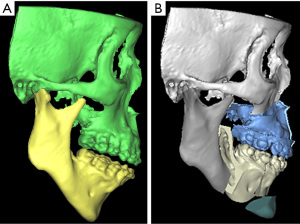
The cohort of Riley and Powell published in 1993 on 306 consecutively treated surgical patients who underwent 2 phases of surgeries, the RDI dropped from 55.8 to 9.2. RDI was also measured with nasal CPAP at 7.2 and was compared with the post-operative RDI for the success rate, which turned out to be 76.5% (42). For patients who underwent phase 2 of surgery, MMA was shown to decrease upper airway collapsibility with the improvement in lateral pharyngeal wall stability being the most prominent (43). Systematic reviews and meta-analyses of post-operative airway changes provided evidence that MMA significantly increases upper airway volume (mean around 7,000 mm3) and cross-sectional area (at least 70 mm2) (37,44). Some quality of life studies were done, and early evaluations (45) to recent findings (46,47) consistently show favorable subjective outcomes.
A systematic review presented that only four major complications were resulted in 455 consecutive patients, with 2 cardiac arrests, one dysrhythmia and one mandibular fracture. The most common complication was facial paresthesia (100%), followed by minor bleeding and infection. The overall minor complication rate was 3.1% if facial paresthesias and malocclusion were not counted. Comparison between orthognathic surgery for dentofacial deformity (DFD) and MMA for OSA patients were made regarding complication rates and no statistically significant difference was found regarding intra-operative complications (48). However, there was significantly more patients in OSA group experiencing post-operative complications than the DFD group. Complications occurring more frequently in OSA patients include dysesthesia/paresthesia, infection, epistaxis, unaesthetic results, TMJ pain, myofascial pain. Other complications include facial nerve injury, wound dehiscence, malocclusion, nonunion, dysphagia, velopharyngeal insufficiency, hemorrhage, medical events, relapse, etc. However, it should be noted that OSA cases are in general much older when compared to those who receive routine orthognathic surgeries, and for those who required MMA are moderate-to-severe cases, who are likely to suffer from more medical co-morbidities from the OSA.
GTA/genioplasty
Genioglossus is a muscle that protrudes and depressed the tongue. It is one of the muscles that dilates the upper airway during sleep. Genioglossal advancement or genioplasty (Figure 4) is indicated in OSA patients exhibiting obstruction at hypoglossal level. It is recently less commonly done as a single procedure and is usually performed simultaneously with surgeries involving other levels. First mentioned in 1984 by Riley and colleagues in conjunction with hyoid bone advancement (49), GTA aims at bringing forward the genioglossus complex at the genial tubercle, giving tension at base of tongue and in turn stabilizes the hypopharyngeal airway and minimizes collapse during sleep. Modifications or subtypes of the procedure have been proposed since then. Techniques include standard sliding genioplasty, inferior sagittal osteotomy/inferior border advancement genioplasty (posteriorly to gonial notch), GTA (“box” surgery), genial bone advancement trephine (GBAT), mortised genioplasty, elliptical window, and trapezoid osteotomy (50-52). Concomitantly, glossoplexy (53) and hyoid bone suspension can be done to augment the results on appropriate cases. Evaluation on the size of chin, length of airway and diagnosis of the dentofacial deformity aids in the clinical decision of choice of the specific approach.
As genioglossal advancement is often performed together with other surgeries, outcome studies targeting solely on surgeries involving the genioglossus complex are sparse. A systematic review and meta-analysis published by Song and colleagues in 2017 compared standard genioplasty, modified genioplasty (detachment of anterior suprahyoid muscles and stretched), GTA alone, and GTA with hyoid suspension (GTA-HS). For isolated genioplasty, isolated GTA, and GTA-HS, the mean AHI differences between pre- and post-operatively were −7.78, −11.1 (from 37.6 to 20.4), and −29.1 (from 34.5 to 15.3), respectively. The corresponding improvements in LSAT were 4.5% (from 82.3% to 86.8%), 2.4% (from 83.1% to 85.5%) and 8.2% (from 80.1% to 88.3%). For ESS, the mean reduction was 5.8 (from 16.5 to 10.7) for isolated genioplasty and 2.9 (from 7.7 to 4.8) for isolated GTA. Improvements on ESS for GTA-HS was not reported. Attention should be paid that there was heterogeneity between the treatment groups regarding pre-operative AHI, variations in techniques, etc. Another study also investigated into the effect of genioglossus advancement and concluded a surgical success of 53% based on the criteria set as AHI <20 and at least 50% reduction (54).
Most of the risks and complications involved in GTA/genioplasty are minor. More common complications include dehiscence (~3%), neurosensory dysfunction (mostly transient and rarely long-term), infection, and bleeding. Careful flap raising and gentle manipulation of the advanced segment are crucial to minimize the changes of having them. The mobilized segment sometimes only included one of the two attachments (~5%) or only part of both genioglossus muscle (~13%) to the genial tubercles. Symmetry and adequate lateral extension in osteotomy are important in prevention (55). Less frequently encountered adverse events are chin ptosis, gingival recession, hardware exposure, mandibular symphyseal fracture, and damage to teeth. Proper closure of mentalis and proper planning of the osteotomy could reduce the risk of these complications (56).
DO
DO is a technique initially used by orthopedic surgeons for malformed legs. One of the first animal studies was done in 1977 by Michieli and Miotti, and was suggested to correct large discrepancies in mandibular micrognathia (57). In recent years, DO has shown its effectiveness in paediatric syndromic patients to prevent or wean off tracheostomy (58). It is also indicated in adult moderate-to-severe OSA patients when routine orthognathic cannot be used, for example, ankylosed temporomandibular joint (58). There are different distraction protocols due to various study results. In general, after osteotomy and placement of distraction device, there are three phases in DO: (I) latency period of 0–7 days for callus formation; (II) distraction period at 0.5–2 mm/day with 1–4 rhythms for production of fibrous tissue and mineralization; and (III) consolidation period of 4–12 weeks depending on the distraction distance, when bone remodeling occurs. Distractors are either external, which are bone-borne percutaneously, or internal, which are bone- or tooth-borne intraorally. The vector of distraction can be unidirectional or multidirectional (59). For non-syndromic mandibular distraction osteogenesis (MDO), a unidirectional internal distractor is usually sufficed. One of the surgical techniques described with bilateral vertical osteotomies performed distal to lower last molar, with the distraction vector directed parallel to the upper occlusal plane to achieve a functional occlusion (60) (Figure 5).
A systematic review in 2016 by Tsui et al. concluded 100% success rate and 82–100% cure rate for adult OSA patients treated with DO (61). Paediatric OSA patients had a success rate of 90–100%. The average distraction distance was 12–29 mm. For adult, the mean AHI dropped from 51.7 to 2.9. LSAT increased from the range of 67% to 77%, to 90.3% to 98.2%. For children, the AHI decreased from preoperative range of 10 to 50, to postoperatively 1.1 to 5. LSAT improved from the range of 73.5% to 93.4%, to 88.9% to 99.2%.
The complications of DO have been the drawback of the technique in adult OSA patients. A systematic review focusing on complications evaluated 1332 patients with acquired deformities and noted an overall complication rate of 43.9%. However, the treatments were heterogenous including in one group, mandibular lengthening, DO in bone grafts, and transport disc DO; and in another group, alveolar DO. Complications in the former group consist of transient neurosensory disturbances (13.4%), infection (5.3%), distraction failure (4.0%), and device-related issues (3.8%) (62). Another systematic review found the complication rate ranges from 0% to 25% and 0% to 20% for adult and children, respectively. Reported complications include infections around the distraction rods, temporary facial nerve palsy, neurosensory disturbances at lower lip and chin areas, anterior open bite, and mechanical failure of distractor. Post-operative tracheostomy was indicated in one child and one death was recorded (61). It is noteworthy that a randomized control trial comparing mandibular DO and bilateral sagittal split osteotomies in non-syndromic adult OSA patients was terminated early due to major complications in the mandibular DO group (63). Effectiveness in treating OSA was demonstrated in both groups, but 1 out of the 9 patients in the mandibular DO group experienced pneumonia, and 2 of them had non-union of the mandible and re-operation was subsequently required for re-fixation and bone grafting. The study attributed the proven distraction protocols were based on younger adult instead older patients as in non-syndromic OSA patients, as older adult has less blood supply and healing capacity. In addition, most mandibular DO patients had to stay in ICU and experienced delayed extubation from surgical swelling (63).
Future development
With the improvements in 3-dimensional imaging and printing, the role of virtual surgical planning (VSP) and patient specific implants (PSI) have significantly improved treatment planning and accuracy in skeletal surgeries for OSA patients. In the modern era of CBCT, individualized computer-aided planning may grant visual determination of osteotomies. This would encourage MMA and GTA/genioplasty and DO. In general, VSP and PSI have advantages over conventional model surgeries for MMA. Not only can 3D simulation be done for estimation of facial changes, but PSI also allows custom-made and printed guides and plates which can shorten surgical time and provides excellent accuracy (64,65). Bony interferences/overlap can be visualized in the virtual plan, allowing operators to be more prepared for the surgery. Regarding GTA, the osteotomy cut can be guided, which ensures inclusion of genial tubercle and capturing the entire genioglossus muscle with symmetry bilaterally. As a side benefit, various data can be measured accurately for research purposes which include but not limit to posterior airway space (PAS) changes, treatment accuracy, and prediction in soft tissue changes.
It appears that there has been more discussion on soft than hard tissue surgeries in recent years. There has been a rise of bariatric surgery prior to other forms of surgeries, multilevel surgery at palate, pharynx, and tongue base, transoral robotic surgery at tongue base, and radiofrequency surgeries (66-68). The DOME technique for narrow and deep vaulted maxilla is one that was more well-known to surgeons. However, more studies done by multiple centers are needed to weigh its benefit and risks involved.
Conclusions
Various skeletal surgeries were proven successful in improving or even curing OSA. In appropriate patients with moderate-to-severe OSA refractory to medical management, bony operations could be considered at an early stage instead of the traditional 2 phases of surgeries. DISE should be performed to identify the areas of obstruction and to implement appropriate treatment strategy. The need for multi-level surgeries could also be determined through DISE and the procedures may be operated at the same time to avoid multiple surgeries. With better understanding of the treatment outcomes, the role of skeletal surgeries in treating OSA is becoming more important.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Sung-Kiang Chuang) for the series “Clinical Outcomes and Innovations in Oral and Maxillofacial Surgery” published in Frontiers of Oral and Maxillofacial Medicine. The article has undergone external peer review.
Peer Review File: Available at https://fomm.amegroups.com/article/view/10.21037/fomm-21-7/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://fomm.amegroups.com/article/view/10.21037/fomm-21-7/coif). The series “Clinical Outcomes and Innovations in Oral and Maxillofacial Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ip MS, Lam B, Lauder IJ, et al. A community study of sleep-disordered breathing in middle-aged Chinese men in Hong Kong. Chest 2001;119:62-9. [Crossref] [PubMed]
- Ip MS, Lam B, Tang LC, et al. A community study of sleep-disordered breathing in middle-aged Chinese women in Hong Kong: prevalence and gender differences. Chest 2004;125:127-34. [Crossref] [PubMed]
- Young T, Shahar E, Nieto FJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med 2002;162:893-900. [Crossref] [PubMed]
- Ahbab S, Ataoglu HE, Tuna M, et al. Neck circumference, metabolic syndrome and obstructive sleep apnea syndrome; evaluation of possible linkage. Med Sci Monit 2013;19:111-7. [Crossref] [PubMed]
- Reutrakul S, Mokhlesi B. Obstructive Sleep Apnea and Diabetes: A State of the Art Review. Chest 2017;152:1070-86. [Crossref] [PubMed]
- Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med 2001;163:19-25. [Crossref] [PubMed]
- Giles TL, Lasserson TJ, Smith BH, et al. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev 2006;CD001106. [PubMed]
- Elfil M, Bahbah EI, Attia MM, et al. Impact of Obstructive Sleep Apnea on Cognitive and Motor Functions in Parkinson's Disease. Mov Disord 2021;36:570-80. [Crossref] [PubMed]
- Weaver TE, Mathias SD, Crosby RD, et al. Relationship between sleep efficacy endpoints and measures of functional status and health-related quality of life in participants with narcolepsy or obstructive sleep apnea treated for excessive daytime sleepiness. J Sleep Res 2021;30:e13210. [Crossref] [PubMed]
- Ulfberg J, Carter N, Talback M, et al. Excessive daytime sleepiness at work and subjective work performance in the general population and among heavy snorers and patients with obstructive sleep apnea. Chest 1996;110:659-63. [Crossref] [PubMed]
- Al-Lawati NM. Sleepy Drivers: High time for action. Sultan Qaboos Univ Med J 2018;18:e127-9. [Crossref] [PubMed]
- Pavwoski P, Shelgikar AV. Treatment options for obstructive sleep apnea. Neurol Clin Pract 2017;7:77-85. [Crossref] [PubMed]
- Rotenberg BW, Murariu D, Pang KP. Trends in CPAP adherence over twenty years of data collection: a flattened curve. J Otolaryngol Head Neck Surg 2016;45:43. [Crossref] [PubMed]
- Mehrtash M, Bakker JP, Ayas N. Predictors of Continuous Positive Airway Pressure Adherence in Patients with Obstructive Sleep Apnea. Lung 2019;197:115-21. [Crossref] [PubMed]
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991;14:540-5. [Crossref] [PubMed]
- Netzer NC, Stoohs RA, Netzer CM, et al. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med 1999;131:485-91. [Crossref] [PubMed]
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008;108:812-21. [Crossref] [PubMed]
- Chung F, Abdullah HR, Liao P. STOP-Bang Questionnaire: A Practical Approach to Screen for Obstructive Sleep Apnea. Chest 2016;149:631-8. [Crossref] [PubMed]
- Chiu HY, Chen PY, Chuang LP, et al. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP, and Epworth sleepiness scale in detecting obstructive sleep apnea: A bivariate meta-analysis. Sleep Med Rev 2017;36:57-70. [Crossref] [PubMed]
- Soares MC, Sallum AC, Goncalves MT, et al. Use of Muller's maneuver in the evaluation of patients with sleep apnea--literature review. Braz J Otorhinolaryngol 2009;75:463-6. [Crossref] [PubMed]
- Jung AR, Koh TK, Kim SJ, et al. Comparison of level and degree of upper airway obstruction by Muller's maneuver and drug-induced sleep endoscopy in obstructive sleep apnea patients. Auris Nasus Larynx 2017;44:571-5. [Crossref] [PubMed]
- Yegïn Y, Celik M, Kaya KH, et al. Comparison of drug-induced sleep endoscopy and Muller's maneuver in diagnosing obstructive sleep apnea using the VOTE classification system. Braz J Otorhinolaryngol 2017;83:445-50. [Crossref] [PubMed]
- Aktas O, Erdur O, Cirik AA, et al. The role of drug-induced sleep endoscopy in surgical planning for obstructive sleep apnea syndrome. Eur Arch Otorhinolaryngol 2015;272:2039-43. [Crossref] [PubMed]
- Koudstaal MJ, Poort LJ, van der Wal KG, et al. Surgically assisted rapid maxillary expansion (SARME): a review of the literature. Int J Oral Maxillofac Surg 2005;34:709-14. [Crossref] [PubMed]
- Liu SY, Guilleminault C, Huon LK, et al. Distraction Osteogenesis Maxillary Expansion (DOME) for Adult Obstructive Sleep Apnea Patients with High Arched Palate. Otolaryngol Head Neck Surg 2017;157:345-8. [Crossref] [PubMed]
- Vale F, Albergaria M, Carrilho E, et al. Efficacy of Rapid Maxillary Expansion in the Treatment of Obstructive Sleep Apnea Syndrome: A Systematic Review With Meta-analysis. J Evid Based Dent Pract 2017;17:159-68. [Crossref] [PubMed]
- Vilani GN, Mattos CT, de Oliveira Ruellas AC, et al. Long-term dental and skeletal changes in patients submitted to surgically assisted rapid maxillary expansion: a meta-analysis. Oral Surg Oral Med Oral Pathol Oral Radiol 2012;114:689-97. [Crossref] [PubMed]
- Vinha PP, Eckeli AL, Faria AC, et al. Effects of surgically assisted rapid maxillary expansion on obstructive sleep apnea and daytime sleepiness. Sleep Breath 2016;20:501-8. [Crossref] [PubMed]
- Iwasaki T, Yoon A, Guilleminault C, et al. How does distraction osteogenesis maxillary expansion (DOME) reduce severity of obstructive sleep apnea? Sleep Breath 2020;24:287-96. [Crossref] [PubMed]
- Carvalho PHA, Moura LB, Trento GS, et al. Surgically assisted rapid maxillary expansion: a systematic review of complications. Int J Oral Maxillofac Surg 2020;49:325-32. [Crossref] [PubMed]
- Li KK. Letter to the editor regarding "Distraction osteogenesis maxillary expansion (DOME) for adult obstructive sleep apnea patients with narrow maxilla and nasal floor" by Yoon et al. Sleep Med 2020;74:298-300. [Crossref] [PubMed]
- Houppermans P, Verweij J, Gooris P, et al. Maxillomandibular advancement in edentulous patients as a treatment option for obstructive sleep apnea: report of two cases and a proposed treatment protocol. Heliyon 2020;6:e03873. [Crossref] [PubMed]
- Riley RW, Powell NB, Guilleminault C, et al. Maxillary, mandibular, and hyoid advancement: an alternative to tracheostomy in obstructive sleep apnea syndrome. Otolaryngol Head Neck Surg 1986;94:584-8. [Crossref] [PubMed]
- Riley RW, Powell NB, Guilleminault C. Obstructive sleep apnea syndrome: a surgical protocol for dynamic upper airway reconstruction. J Oral Maxillofac Surg 1993;51:742-7; discussion 8-9. [Crossref] [PubMed]
- Liu SY, Awad M, Riley RW. Maxillomandibular Advancement: Contemporary Approach at Stanford. Atlas Oral Maxillofac Surg Clin North Am 2019;27:29-36. [Crossref] [PubMed]
- Wei S, Zhang Y, Guo X, et al. Counterclockwise maxillomandibular advancement: a choice for Chinese patients with severe obstructive sleep apnea. Sleep Breath 2017;21:853-60. [Crossref] [PubMed]
- Louro RS, Calasans-Maia JA, Mattos CT, et al. Three-dimensional changes to the upper airway after maxillomandibular advancement with counterclockwise rotation: a systematic review and meta-analysis. Int J Oral Maxillofac Surg 2018;47:622-9. [Crossref] [PubMed]
- Kim T, Kim HH, Hong S, et al. Change in the Upper Airway of Patients With Obstructive Sleep Apnea Syndrome Using Computational Fluid Dynamics Analysis: Conventional Maxillomandibular Advancement Versus Modified Maxillomandibular Advancement With Anterior Segmental Setback Osteotomy. J Craniofac Surg 2015;26:e765-70. [Crossref] [PubMed]
- Yang YL, Hsu SSP, Lin CCH. Segmental Maxillomandibular Rotational Advancement to Correct Obstructive Sleep Apnea in a Patient Skeletal Class II malocclusion - A Case Report. Taiwanese Journal of Orthodontics 2020;29.
- Goh YH, Lim KA. Modified maxillomandibular advancement for the treatment of obstructive sleep apnea: a preliminary report. Laryngoscope 2003;113:1577-82. [Crossref] [PubMed]
- Wu Q, Wang Y, Wang P, et al. The inverted-L ramus osteotomy versus sagittal split ramus osteotomy in maxillomandibular advancement for the treatment of obstructive sleep apnea patients: A retrospective study. J Craniomaxillofac Surg 2019;47:1839-47. [Crossref] [PubMed]
- Riley RW, Powell NB, Guilleminault C. Obstructive sleep apnea syndrome: a review of 306 consecutively treated surgical patients. Otolaryngol Head Neck Surg 1993;108:117-25. [Crossref] [PubMed]
- Li KK, Guilleminault C, Riley RW, et al. Obstructive sleep apnea and maxillomandibular advancement: an assessment of airway changes using radiographic and nasopharyngoscopic examinations. J Oral Maxillofac Surg 2002;60:526-30; discussion 31. [Crossref] [PubMed]
- Shokri A, Ramezani K, Afshar A, et al. Upper Airway Changes Following Different Orthognathic Surgeries, Evaluated by Cone Beam Computed Tomography: A Systematic Review and Meta-analysis. J Craniofac Surg 2021;32:e147-52. [Crossref] [PubMed]
- Lye KW, Waite PD, Meara D, et al. Quality of life evaluation of maxillomandibular advancement surgery for treatment of obstructive sleep apnea. J Oral Maxillofac Surg 2008;66:968-72. [Crossref] [PubMed]
- Lin CH, Chin WC, Huang YS, et al. Objective and subjective long term outcome of maxillomandibular advancement in obstructive sleep apnea. Sleep Med 2020;74:289-96. [Crossref] [PubMed]
- Tsui WK, Yang Y, McGrath C, et al. Improvement in quality of life after skeletal advancement surgery in patients with moderate-to-severe obstructive sleep apnoea: a longitudinal study. Int J Oral Maxillofac Surg 2020;49:333-41. [Crossref] [PubMed]
- Passeri LA, Choi JG, Kaban LB, et al. Morbidity and Mortality Rates After Maxillomandibular Advancement for Treatment of Obstructive Sleep Apnea. J Oral Maxillofac Surg 2016;74:2033-43. [Crossref] [PubMed]
- Riley R, Guilleminault C, Powell N, et al. Mandibular osteotomy and hyoid bone advancement for obstructive sleep apnea: a case report. Sleep 1984;7:79-82. [Crossref] [PubMed]
- Powers DB, Allan PF, Hayes CJ, et al. A review of the surgical treatment options for the obstructive sleep apnea/hypopnea syndrome patient. Mil Med 2010;175:676-85. [Crossref] [PubMed]
- Lee NR, Madani M. Genioglossus muscle advancement techniques for obstructive sleep apnea. Atlas Oral Maxillofac Surg Clin North Am 2007;15:179-92. [Crossref] [PubMed]
- Goh YH, Abdullah V, Kim SW. Genioglossus Advancement and Hyoid Surgery. Sleep Med Clin 2019;14:73-81. [Crossref] [PubMed]
- Vargo JD, Ogan WS, Tanna N, et al. Modified Genioglossal Advancement for Isolated Treatment of Obstructive Sleep Apnea. J Craniofac Surg 2017;28:1274-7. [Crossref] [PubMed]
- Kuscu O, Suslu AE, Ozer S, et al. Sole effect of genioglossus advancement on apnea hypopnea index of patients with obstructive sleep apnea. Acta Otolaryngol 2015;135:835-9. [Crossref] [PubMed]
- Camacho M, Liu SY, Certal V, et al. Large maxillomandibular advancements for obstructive sleep apnea: An operative technique evolved over 30 years. J Craniomaxillofac Surg 2015;43:1113-8. [Crossref] [PubMed]
- Genioglossus Cheng A, Advancement Genioplasty. Atlas Oral Maxillofac Surg Clin North Am 2019;27:23-8. [Crossref] [PubMed]
- Michieli S, Miotti B. Lengthening of mandibular body by gradual surgical-orthodontic distraction. J Oral Surg 1977;35:187-92. [PubMed]
- Leung YY, Lai KKY. Management of obstructive sleep apnoea: an update on the role of distraction osteogenesis. Curr Opin Otolaryngol Head Neck Surg 2018;26:214-20. [Crossref] [PubMed]
- Hariri F, Chin SY, Rengarajoo J, et al. Distraction Osteogenesis in Oral and Craniomaxillofacial Reconstructive Surgery. Osteogenesis and Bone Regeneration. 2018.
- Ow A, Cheung LK. Bilateral sagittal split osteotomies and mandibular distraction osteogenesis: a randomized controlled trial comparing skeletal stability. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010;109:17-23. [Crossref] [PubMed]
- Tsui WK, Yang Y, Cheung LK, et al. Distraction osteogenesis as a treatment of obstructive sleep apnea syndrome: A systematic review. Medicine (Baltimore) 2016;95:e4674. [Crossref] [PubMed]
- Verlinden CR, van de Vijfeijken SE, Tuinzing DB, et al. Complications of mandibular distraction osteogenesis for acquired deformities: a systematic review of the literature. Int J Oral Maxillofac Surg 2015;44:956-64. [Crossref] [PubMed]
- Tsui WK, Yang Y, McGrath C, et al. Mandibular distraction osteogenesis versus sagittal split ramus osteotomy in managing obstructive sleep apnea: A randomized clinical trial. J Craniomaxillofac Surg 2019;47:750-7. [Crossref] [PubMed]
- Hsu SS, Gateno J, Bell RB, et al. Accuracy of a computer-aided surgical simulation protocol for orthognathic surgery: a prospective multicenter study. J Oral Maxillofac Surg 2013;71:128-42. [Crossref] [PubMed]
- Yang WF, Choi WS, Leung YY, et al. Three-dimensional printing of patient-specific surgical plates in head and neck reconstruction: A prospective pilot study. Oral Oncol 2018;78:31-6. [Crossref] [PubMed]
- Cambi J, Chiri ZM, De Santis S, et al. Outcomes in single-stage multilevel surgery for obstructive sleep apnea: Transoral robotic surgery, expansion sphincter pharyngoplasty and septoplasty. Int J Med Robot 2019;15:e2034. [Crossref] [PubMed]
- Mulholland GB, Jeffery CC, Ziai H, et al. Multilevel Palate and Tongue Base Surgical Treatment of Obstructive Sleep Apnea: A Systematic Review and Meta-analysis. Laryngoscope 2019;129:1712-21. [Crossref] [PubMed]
- Patel A, Kotecha B. Minimally Invasive Radiofrequency Surgery in Sleep-Disordered Breathing. Healthcare (Basel) 2019;7:97. [Crossref] [PubMed]
Cite this article as: Wan CCJ, Leung YY. The role of skeletal surgical treatment in the management of obstructive sleep apnea: an overview. Front Oral Maxillofac Med 2022;4:18.