Systematic review of platelet-rich fibrin (PRF) centrifugation protocols in oral and maxillofacial surgery and the introduction of AR2T3: an easy to remember acronym to correctly report vertical and horizontal PRF centrifugation
Introduction
Blood is a complex organ and it is constituted of three main cell components in variable proportions, namely, red blood cells (≈43%), white blood cells (≈1%) and platelets (≈1%) suspended in plasma (≈55%). By applying centrifugal force through acceleration, these cells can be separated from plasma. The effect of centrifugation on cells depends on their different mass, which is the product of their volume and density (density of erythrocytes: 1,090–1,100 kg/m3, leukocytes; 1,050–1,085 kg/m3, platelets 1,040–1,060 kg/m3, plasma 1,025 kg/m3) (1). Consequently, due to the higher mass of the erythrocytes, when blood is centrifuged, erythrocytes settle faster at the bottom of a recipient, while leukocytes and platelets deposit on top of the erythrocytes, followed by plasma over the cells (2).
The speed of acceleration exerted to blood through centrifugation is known as the relative centrifugation force (RCF) and it is expressed in multiples of earth’s gravitational force (9.8.1 m/s2) and abbreviated as ‘‘g’’. Thus, the higher the mass of cells and the RCF applied, the faster cells will settle at the bottom of a tube. The speed in which suspended cells move outside of the blood plasma and sediment at the bottom of the tubes is known as sedimentation (3). The speed of sedimentation is dependent on cells volume and the difference between the density of cells and the density of plasma when the gravitational force is applied. In other words, the sedimentation speed of cells will decrease as the density of plasma increases and will increase as the RCF increases. As blood is an organ highly influenced by age, gender, nutrition, liquid intake and systemic health, the quantity of cells in blood and its viscosity can vary within each individual patient, consequently, resulting in changes in the ratio of fractioning after centrifugation (4,5).
The aforementioned principles apply to all blood derivates used to improve wound healing, i.e., platelet concentrates (6). Platelet-rich fibrin (PRF) is a second-generation cell concentrate derived from human blood containing platelets, white blood cells, stem-like cells and growth factors (6,7). It is obtained through a one-step centrifugation and since its introduction in dentistry and maxillofacial surgery in 2001, has found many applications (7). A recent review involving 72 studies and 1,822 patients in total described the applications of PRF in the fields of endodontic, implantology, maxillofacial surgery, orthodontics and periodontology. 38 from these studies, involving 70% of the patients treated, were conducted with a high scientific rigor (randomized and controlled prospect studies) and showed that PRF is a beneficial tool that significantly improves bone and soft tissue regeneration (7).
According to the tubes used to collect blood, a solid/clot PRF or a liquid/injectable PRF can be obtained (8,9). The centrifugation process and the surface of the tubes initiates the coagulation cascade of blood and activates platelets, causing bioactive molecules (hemostatic factors, angiogenic factors, growth factors, proteases, serotonin) to be released from the alpha and dense granules (10). In the case of solid/clot PRF, a glass tube provides a well-established clot after centrifugation since the coagulation cascade is activated by the contact of blood with glass due to its negatively charged surface (11,12). On the contrary, for a liquid/injectable PRF a plastic tube is required. The plastic tubes cause the slow activation of prekallikrein (PK)—Factor XII complex, providing the clinicians a mean of 12.95 minutes after centrifugation for the liquid/injectable PRF to coagulate (11,13). The final product after centrifugation consists of a fibrin matrix, in which platelets are trapped. Previous studies have shown that the mean concentration of platelets in PRF is five times the amount of undivided blood (14). Thus, its use in the clinical setting, provides a quick source of a high concentration of bioactive molecules that promote wound healing (15).
White blood cells are also found within the PRF. White blood cells can be divided in 5 categories: neutrophils, monocytes, eosinophils, basophils and lymphocytes. Neutrophils as effector cells of the innate immune system, make up the majority of white blood cells (40–80%) and are densely packed with secretory granules (16,17). A recent study that intended to characterize PRF, showed the presence of B- and T-lymphocytes, monocytes, progenitor cells and neutrophilic granulocytes after centrifugation within solid/clots PRF and liquid/injectable PRF (18). The binding of platelets, neutrophils and monocytes increases through centrifugation. This raises degranulation of the inflammatory cytokines interleukin 1-Beta (IL-1β) and interleukin 8 (IL-8) (19), as well as the pro-wound healing cytokines interleukin 4 (IL-4) and vascular endothelial growth factor (VEGF) (20).
In the literature, diversities of centrifugation protocols (CP) to obtain PRF are described. Recent published studies have shown that the protocol of centrifugation and the centrifuge machine used affect the final characteristic of PRF (6,21). Furthermore, preclinical a clinical research in dentistry and maxillofacial surgery describes augmented healing properties (e.g., Increased bone regeneration and vascular formations) by adjusting the g-force to specific indications (7-9,18). It is therefore crucial to report without omission all parameters to produce PRF in order to obtain reproducible results. The aim of this review article is to revise the existence of standardization in the report of the latest protocols to produce PRF. I present the following article in accordance with the PRISMA reporting checklist (available at https://fomm.amegroups.com/article/view/10.21037/fomm-21-40/rc).
Methods
Search strategy
To reduce bias of selection, a systematic approach was used to perform the electronic literature search following the steps described by Egger et al. (22). The research question was formulated as follow: ‘‘Is the reporting of PRF’s centrifugation protocols standardized?’’ conforming to the FINER framework (Table 1). The electronic search was conducted in the biomedical search engine “National Library of Medicine” (PubMed-MEDLINE) with a combination of Medical Subject Heading search terms (i.e., MeSH) together with Boolean operators to further refine the search (Table 2). Articles that fulfilled the inclusion criteria were those performed in humans in the fields of dentistry and oral and maxillofacial surgery, articles written in English and those including the word “platelet-rich fibrin” or “PRF” in the title. No limitations were set regarding the publication year. The first search was conducted on March 2020 and a second search was conducted on October 2020 for horizontal centrifugation protocols. The full texts of the selected articles were examined by the author and the data was extracted using extraction sheets designed for this review. Previous published reviews were not included in the study but were used to identify potentially relevant information. References of the selected articles were scrutinized to search for the description of the PRF’s protocol and its clinical application. The following information was extracted: Name of first author, year of publication and the protocol to obtain solid/clot or liquid/injectable PRF as follows: revolutions per minute-rpm, time of centrifugation, RCF (×g), radius of the rotor measured from the axis of the rotor to the base of the tube (RCF-max), name of the centrifuge machine, angle of centrifugation and the characteristics of the tubes. Additionally, articles describing the use of PRF as the only treatment in maxillofacial surgery were selected and the diagnostics of the involved subjects and the clinical results were recorded. A manual search was performed in the following relevant journals of oral and maxillofacial surgery: International Journal of Oral and Maxillofacial Surgery, Journal of Oral and Maxillofacial Surgery, Journal of Cranio-Maxillofacial Surgery, Oral and Maxillofacial Surgery Clinics of North America, Oral Surgery Oral Medicine Oral Pathology Oral Radiology and British Journal of Oral & Maxillofacial Surgery. The results were gathered in table format, analyzed and discussed. A flow chart describing the search methodology was depicted using the PRISMA template (Figure 1) (23).
Table 1
| Steps | General activities | Specific activities |
|---|---|---|
| I | Formation of working group | Maxillofacial surgeon with experience in blood concentrates and writing systematic literature reviews |
| II | Formulation of the review question | ‘‘Is the reporting of PRF’s centrifugation protocols standardized?’’ |
| III | Identification of relevant studies on PubMed, Cochrane and Key Journals | (I) Selection of Keywords |
| (II) Use of Boolean operators (AND; OR; NOT) | ||
| (III) Search (Table 2 Search strategy) | ||
| (IV) Inclusion criteria | ||
| (V) Elimination of duplicates | ||
| (VI) Manual search | ||
| IV | Analyses and presentation | All data is shown in tables and graphs |
Adapted from Herrera-Vizcaino C, Albilia JB. Front Oral Maxillofac Med, 2021 (15). PRF, platelet-rich fibrin.
Table 2
| Search strategy | Database |
|---|---|
| Systematic search | |
| Keywords: (((Platelet-rich fibrin) OR (PRF)) AND (dentistry)) | PubMed |
| Hand search | |
| International Journal of Oral and Maxillofacial Surgery, Journal of Oral and Maxillofacial Surgery, Journal of Cranio-Maxillofacial Surgery, Oral and Maxillofacial Surgery Clinics of North America, Oral Surgery Oral Medicine Oral Pathology Oral Radiology and British Journal of Oral & Maxillofacial Surgery | – |
Results
Many studies fulfilled the inclusion criteria but were excluded because they were performed in animals and/or other medical fields. A total of 121 articles were found in the literature to be eligible for qualitative evaluation (Figure 1). The search showed the increment of published articles beginning in 2016 and the highest pick in 2018 with 39 articles. The search gave a result of 29 different protocols of PRF involving solid/clot and liquid/injectable versions (Tables 3-7). Sixteen centrifuge machines were mentioned in the reviewed articles; however, the characteristics of the centrifuge machines were not always available in the literature found (Table 8). Additional information was gathered from the manufacturers. Our analysis of the different centrifuges revealed that the angulations of the tube within the fixed-angle centrifuge machines range from 33° to 45° and the radius maximum (r-max—distance from the center of rotation to the bottom of the tubes) from 85 to 132 mm.
Table 3
| Published studies | Protocols/centrifuge |
|---|---|
| Moussa et al. [2016] (24) | Protocols: 3,500 rpm ×12–15 min |
| Centrifuge: Centrifuge 800, China (24) | |
| Dohan et al. [2006] (25), Aroca et al. [2009] (26), Sharma et al. [2011; 2011] (27,28), Simonpieri et al. [2011] (29), Tatullo et al. [2012] (30), Jankovic et al. [2012] (31), Singh et al. [2013] (32), Bajaj et al. [2013] (33), Bansal et al. [2013] (34), Suttapreyasri et al. [2013] (35), Geeta et al. [2013] (36), Soydan et al. [2014] (37), Joseph et al. [2014] (38), Yelamali et al. [2015] (39), Kumar et al. [2015] (40), Inchingolo et al. [2015] (41), Dhiman et al. [2015] (42), Hamzacebi et al. [2015] (43), Boora et al. [2015] (44), Shawky et al. [2016] (45), Ajwani et al. [2015] (46), Mathur et al. [2015] (47), Pradeep et al. [2015] (48), Sandhu et al. [2015] (49), Pradeep et al. [2016] (50), Doiphode et al. [2016] (51), Munoz et al. [2016] (52), Kanayama et al. [2016] (53), Kumar et al. [2016] (54), Subash et al. [2016] (55), Asaka et al. [2017] (56), Park et al. [2017] (57), Kanoriya et al. [2017] (58), Varghese et al. [2017] (59), Bilginaylar [2017] (60), Zhang et al. [2017] (61), Gülşen et al. [2017] (62), Goel et al. [2017] (63), Cömert Kılıç et al. [2017] (64), Pripatnanont et al. [2017] (65), Bajaj et al. [2017] (66), Patel et al. [2017] (67), Sharma et al. [2018] (68), Afat et al. [2018] (69), Eshghpour et al. [2018] (70), Asmael et al. [2018] (71), Bahammam et al. [2018] (72), Pichotano et al. [2018] (73), Pinto et al. [2018] (74), Zhou et al. [2018] (75), Unsal et al. [2018] (76), Santhakumar et al. [2018] (77), Nageh et al. [2018] (78), Mahajan et al. [2018] (79), Bilginaylar [2019] (80), Lv et al. [2018] (81), Mazzone et al. [2018] (82), Caymaz et al. [2019] (83), Diana et al. [2018] (84) | Protocols: 3,000 rpm ×10 min |
| Centrifuges: (I) EBA 20 Andreas Hettich, Tuttlingen, Germany (26,35,61); (II) PC-02; Process, France (29,43); (III) REMI Laboratories, India (33,42,46-48,50,55,59,63,67); (IV) Almicro™ Instruments, India (38); (V) Centrifugette model 4206, ALC, France (41); (VI) Elektro-mag M415P, Turkey (60,80,83); (VII) NUVE NF 200, Turkey (62); (VIII) Labofuge centrifuge, Heraeus Kulze (65,70,74); (IX) Centrifuge Xiangtian, Jiangsu China (71); (X) Kasvi K140815, Curitiba, Brazil (73); (XI) Nüve Laboratory Equipments, NF200, Turkey (76); (XII) Not reported (25,27,28,30-32,34,36,37,39,40,44,45,49,51-54,56,58,64,66,68,69,72) and (75,77-79,81,82,84) | |
| Chang et al. [2011] (85), Bains et al. [2012] (86), Dar et al. [2016] (87), Khan et al. [2018] (88), Thorat et al. [2017] (89), Ragab et al. [2019] (90) | Protocols: 3,000 rpm ×12 min |
| Centrifuge: (I) PC-02, Process, France (85); (II) REMI Laboratories, India (86,88,89); (III) Centrifuge Model 801, China (90); (IV) Not reported (87) | |
| Kizildağ et al. [2018] (91) | Protocols: 3,000 rpm ×10 min |
| Centrifuge: EBA 20, Hettich Centrifuges, Germany (91) | |
| Al-Ahmady et al. [2018] (92) | Protocols: 3,000 rpm ×20 min |
| Centrifuge: Not reported (92) | |
| Cortese et al. [2016] (93) | Protocols: 3,000 rpm ×13 min |
| Centrifuge: Not reported (93) |
Table 4
| Published studies | Protocols/centrifuge |
|---|---|
| Ustaoğlu et al. [2016] (94), Arabaci et al. [2017] (95), Tabrizi et al. [2018] (96), Pirebas et al. [2018] (97), Daugela et al. [2018] (98) | Protocols: 2,800 rpm ×12 min |
| Centrifuge: (I) IntraSpin centrifuge Boca Raton, FL, USA (96); (II) Mikro 22 R Hettich Centrifugal Machine, Tuttlingen, Germany (97,98); (III) EBA 20, Andreas Hettich (98); (IV) Not reported (94,95) | |
| Sammartino et al. [2011] (99) | Protocols: 2,700 rpm ×18 min |
| Centrifuge: Centrifuge PRF DUO Process, France (99) | |
| Ruga et al. [2011] (100), Marenzi et al. [2015] (101), Öncü et al. [2015] (102), Kotsakis et al. [2016] (103), Bakhtiar et al. [2017] (104), Kapustecki et al. [2016] (105), Anwandter et al. [2016] (106), Gönen et al. [2017] (107), Gurler et al. [2016] (108), Cortese et al. [2016] (93), Temmerman et al. [2016] (109), Öncü et al. [2017] (110), Sezgin et al. [2017] (111), Ozcan et al. [2017] (112), Patidar et al. [2017] (113), Pinto et al. [2017] (114), Uzun et al. [2018] (115), Shah et al. [2017] (116), Betancourt et al. [2017] (117), Culhaoglu et al. [2018] (118), Asutuay et al. [2017] (119), Temmerman et al. [2018] (120), S Medikeri et al. [2018] (121), Dixit et al. [2018] (122), Liang et al. [2018] (123) | Protocols: 2,700 rpm ×12 min |
| Centrifuge: (I) Centrifuge PRF DUO Process, France (100,102,106); (II) PC-02, process, France (101,110,111,118); (III) Intra-Spin, Boca-Raton, FL, USA (101,104,108,109,114,120,124); (IV) Nüve Laboratory Equipments, NF200, Ankara, Turkey (115); (V) Hettich Centrifugal Machine, Tuttlingen, Germany (112); (VI) Labo-fuge® 300; Heraus GmbH, Hanau, Germany (117); (VII) REMI-8C, Laboratory (121); (VIII) Not reported (93,103,105,107,113,116,119,122,123) | |
| Cortellini et al. [2018] (124) | Protocols: 2,700 rpm/408 g ×12 min |
| Centrifuge: Intra-Spin, Boca-Raton, FL, USA. r-max 5 cm (124) | |
| Cortellini et al. [2018] (124) | Protocols: 2,700 rpm ×3 min |
| Centrifuge: Intra-Spin, Boca-Raton, FL, USA. r-max 5 cm (124) | |
| Choukroun et al. [2006] (125), Gamal et al. [2016] (126) | Protocols: 2,500 rpm (about 280 g) ×10 minutes |
| Centrifuge: (I) Centrifuge PRF DUO Process, France (126); (II) Not reported (125) |
Table 5
| Published studies | Protocols/centrifuge |
|---|---|
| Lei et al. [2019] (127), Caymaz et al. [2019] (83) | Protocols: 1,500 rpm ×14 min |
| Centrifuge: (I) Centrifuge PRF DUO Process, France (127); (II) Elektro-mag M415P, Istanbul, Turkey (83) | |
| Demetoglu et al. [2018] (128) | Protocols: 1,500 rpm ×8 min |
| Centrifuge: Not reported (128) | |
| Nørholt et al. [2016] (129) | Protocols: 1,300 rpm ×14 min |
| Centrifuge: Centrifuge PRF DUO Process, France (129) | |
| Chenchev et al. [2017] (130), Fortunato et al. [2018] (131) | Protocols: 1,300 rpm ×8 min |
| Centrifuge: Centrifuge PRF DUO Process, France (130,131) | |
| Chenchev et al. [2017] (130), Fortunato et al. [2018] (131) | Protocols: 700 rpm ×3 min |
| Centrifuge: Centrifuge PRF DUO Process, France (130,131) |
Table 6
| Published studies | Protocols/centrifuge |
|---|---|
| Lekovic et al. [2012] (132) | Protocols: 1,000 g ×10 min |
| Centrifuge: Labofuge 300; Heraus GmbH, Hanau, Germany (132) | |
| Olgun et al. [2018] (133) | Protocols: 878 g ×12 min |
| Centrifuge: Mikro 22R Hettich Centrifugal Machine, Tuttlingen, Germany (133) | |
| Choukroun and Ghanaati [2018] (134) | Protocols: 710 g ×8 min |
| Centrifuge PRF DUO Process, France (134) | |
| Meza et al. [2019] (135) | Protocols: 500 g ×12 min |
| Centrifuge: Intra-Spin, Boca-Raton, FL, USA (135) | |
| Thorat et al. [2011] (136), Peck et al. [2011] (137), Agarwal et al. [2014] (138), Bolukbasi et al. [2015] (139), Nizam et al. [2018] (140), de Almeida Barros Mourão et al. [2018] (141) | Protocols: 400 g ×12 min |
| Centrifuge: (I) PLC-03, Hi-care International, Taiwan (137); (II) Centrifuge PRF DUO Process, France (139); (III) Nüve Laboratory Equipment, Turkey (140); (IV) Montserrat®, São Paulo, Brazil (141); (V) Not reported (136,138) | |
| Lorenz et al. [2018] (142) | Protocols: 208 g ×8 min |
| Centrifuge: Centrifuge PRF DUO Process, France (142) | |
| Nacopoulos et al. [2019] (143) | Protocols: 208 g ×5 min |
| Centrifuge: Centrifuge PRF DUO Process, France (143) | |
| Choukroun and Ghanaati [2018] (134) | Protocols: 177 g ×8 min |
| Centrifuge PRF DUO Process, France (134) | |
| Lorenz et al. [2018] (142), Nacopoulos et al. [2019] (143) | Protocols: 60 g ×3 min |
| Centrifuge: Centrifuge PRF DUO Process, France (142) | |
| Choukroun and Ghanaati [2018] (134) | Protocols: 44 g ×8 min |
| Centrifuge PRF DUO Process, France (134) |
Table 7
Table 8
| Centrifuges | Radius (r-max) | Fixed-angle/horizontal |
|---|---|---|
| PC-02, Process, France | 88 mm | Fixed-angle—33° |
| Intra-Spin Boca-Raton, FL, USA | 85 mm | Fixed-angle—33° |
| Centrifuge PRF DUO Process, France | 110 mm | Fixed-angle—41.3° |
| EBA 200 Centrifuge, Andreas Hettich, Tuttlingen, Germany | ≈86 mm | Fixed-angle—33° |
| PLC-03, Hi-care International, Taiwan | NR | NR |
| Labofuge 300; Heraus GmbH, Hanau, Germany | NR | NR |
| REMI Laboratories, India | NR | NR |
| Almicro™ Instruments, India | NR | NR |
| Centrifuge 800, China | NR | NR |
| Elektro-mag M415P, Istanbul, Turkey | 90 mm | Fixed angle—35° |
| Nüve Laboratory Equipments, NF200, Turkey | 101 mm | NR |
| Mikro 22R Hettich Centrifugal Machine, Germany | ≈86 mm | Fixed angle—35° |
| Kasvi K140815, Curitiba, Brazil | 103 mm | Fixed-angle—36° |
| Montserrat®, São Paulo, Brazil (Modelo 80-2B) | 130 mm | Fixed-angle—45° |
| Eppendorf 5702 horizontal centrifuge (Hamburg, Germany) | 132 mm | Horizontal |
| Centrifuge Xiangtian, Jiangsu China | NR | NR |
Data was extracted from the analyzed papers and from the manufacturer’s information. NR, not reported.
Protocols of centrifugation reported with RPM
Protocols of centrifugation in a high range of RPM [3,500–2,900]
A total of 70 articles described a PRF protocol using an RPM within this range. For the solid/clot PRF, the protocol found in the literature with the highest RPM was “3,500 rpm ×12–15 min’’. Only one study was found using this protocol (Table 3). The name of the centrifuge machine was mentioned with no additional characteristics (24). Sixty [60] studies described the protocol ‘‘3,000 rpm for 10 minutes’’, which made it the most cited protocol in the high range of RPM. From these 60 studies, only 29 named the centrifuge machine used. In total, 12 different centrifuge machines were used to produce PRF (Table 3). The second most cited protocol in this range was “3,000 rpm ×12 min” (85-90). Five studies reported two different centrifuge machines and one study did not report which centrifuge was used (87). In this group, no further characteristics of the centrifuge machines were mentioned. Four additional protocols using 3,000 rpm were found with a variation in the time of centrifugation (92-94). The search did not show protocols used to obtain liquid/injectable PRF within this range. All the studies reported the time of centrifugation, but none described the RCF-min/clot/max, the r-max or the angle of centrifugation. In this range of RPM, the study performed by Kizildağ et al. (91) was the only study reporting the protocol of centrifugation using RPM and RCF.
Protocols of centrifugation in a middle range of RPM (2,800–1,600)
A total of 33 articles described a PRF protocol using an RPM within this range (Table 4). For the solid/clot PRF, the protocol described with the highest rpm was “2,800 rpm ×12 min’’ obtained with 3 different centrifuge machines (94–98). Two studies reported the protocol of centrifugation but did not report the centrifuge machine used (94,95). No additional characteristics of the centrifuge machines were reported. The most cited protocol in this range was “2,700 rpm ×12 min’’ with 24 studies. Seven different centrifuge machines were used to prepare PRF with this protocol. From the 29 studies, 9 did not report the centrifuge machine used (93,103,105,107,113,116,119,122,123). A second study reporting a protocol with 2700 rpm was found but with a centrifugation time of 18 minutes (99). Notably, only one study reported additionally the r-max and the RCF (“2,700 rpm/408 g ×12 min’’ and r-max 5 cm) (124). Three additional studies reported the protocol of centrifugation using RPM and RCF—“2,500 rpm (about 280 g) ×10 minutes’’ (125,126). Furthermore, one study was found reporting a liquid/injectable PRF protocol (2,700 rpm ×3 min) in this range of centrifugation with a considerable lower time (124). Similarly, to the aforementioned range, all of the studies reported the time of centrifugation, but only one described the RCF and the r-max of the rotor (124). None of the studies reported the angle of centrifugation.
Protocols of centrifugation in a low range of RPM [1,500–700]
A total of 6 articles described a PRF protocol using RPM within this range (83,127-131) (Table 5). For the solid/clot PRF, the most cited protocol found was “1,500 rpm × 14 min’’. Two studies were found using this protocol. The search showed an additional protocol using 1,500 rpm but with 8-min centrifugation. This study did not report the centrifuge machine used (128). Three additional studies reported a protocol using the same RPM, centrifuge machine but a different time of centrifugation [“1,300 rpm ×14 min’’ (129) and “1,300 rpm ×8 min’’ (130,131)]. Additionally, three studies reported two different protocols to obtain liquid/injectable PRF within this range. The protocol with the highest RPM was “1,300 rpm ×5 min’’ (143) followed by a protocol with a lower RPM and time of centrifugation—“700 rpm ×3 min’’ (130,131). One of the studies from the liquid/injectable PRF reported the RCF (×g) and the r-max of the centrifuge (143). All the studies reported the time of centrifugation; one described the RCF (×g) and the r-max, but none of the studies reported the angle of centrifugation.
Protocols of centrifugation reported with g-force
Protocols of centrifugation in a high (1,000–700 g)/ middle (500–208 g)/ low (60–44 g) range of g-force
A total of 13 articles described a PRF protocol using g-force (Tables 6,7). Two from these did not report the centrifuge machine used (136,138). Two studies described two different protocols of centrifugation of solid/clot PRF in the high range using a fixed-angle centrifuge (132,133) or horizontal centrifugation (144). The protocol with the highest g-force was “1,000 g × 10 min’’ (132). The three studies reported the name of the centrifuge machine without further characteristics. In the middle range of centrifugation, 10 studies reported 4 different protocols of centrifugation. The highest protocol reported was 500 g × 12 min (135). Six studies reported using 400 g with 12 min of centrifugation and one with 8 min. Four different centrifuge machines were named in this range to produce PRF. Although two studies reported the use of 280 g for the CP, the centrifugation time differed (142,143). In high and middle range, none of the protocols were used to produce liquid/injectable PRF. In the low range, three studies were found reporting the protocols 60 g × 3 min and 44 g × 8 min. to produce liquid/injectable PRF (134,142,143). Both studies named the same centrifuge machine without further characteristics. All the studies reported the time of centrifugation and only one study reported the radius of the rotor of the centrifuge machine (143).
Time of centrifugation
The articles included in the review described the time (min) used for the centrifugation of PRF with a broad variability. The highest times reported were 20 and 18 min of centrifugation. These times of centrifugation were reported by only 2 articles (92,99). A similar exception was also observed with 15 min and 13 min of centrifugation with only 2 articles reporting its use (24,93). The most reported times of centrifugation to obtain solid PRF were 14 min in 2 protocols (2 articles), 12 min in 9 protocols (44 articles), 10 min in 4 protocols (64 articles) and 8 min in 6 protocols (3 articles) (Tables 4-7). The protocols with lower times of centrifugation were implemented to obtain liquid PRF. The lowest time used were 5 min in 1 article and 3 min in 4 articles.
Tube’s size and material
In general, the solid/clot PRF are prepared with empty glass-coated tubes and silica-coated plastic tubes. The liquid/injectable PRF are prepared with empty plastic tubes without additive. Additionally, 4 studies were found describing the use of titanium tubes to prepare solid/clot PRF (94,97,115,133). The search also showed a variety of tube sizes used to produce PRF. The size most commonly used was 10mL, nonetheless, different sizes were found ranging from 5 to 15 mL tubes.
Clinical applications of PRF in oral and maxillofacial surgery
In total, 43 studies were found reporting to have used PRF as a sole treatment. From these, 29 were controlled studies compared to natural wound healing (https://cdn.amegroups.cn/static/public/fomm-21-40-1.pdf). The pathologies treated were socket preservation, implant stability and bone regeneration, reduction of pain and swelling, sinus lift, management of bisphosphonate-related osteonecrosis of the jaw, closure of oroantral communication, filling of bone defects with PRF after cyst debridement, enhancement of soft tissue healing and management of pain, haemostatic control of patients with anticoagulant oral therapy and bone augmentation procedures. Forty studies reported positive outcomes with statistical improvement in the groups treated with PRF. PRF was implemented the highest to enhance soft tissue healing (8 articles), and to increase implant stability by inducing bone regeneration (7 articles). Three studies investigating the reduction of pain and swelling implemented CPs in the high (3,000 rpm × 10 min) and middle range (2,700 rpm × 12 min) and did not observed statistically significant reduction of pain between the PRF and control groups (62,69,119). However, one additional study using a CP in the high range reported statistically significant reduction of pain and swelling the first post-operative day compared to control (40). 24 CPs reported in RPM and 2 in RFC (×g) were implemented within the high, middle and low range. The treatment with the highest diversity of CPs [RPM/RCF (×g)/time] was the enhancement of soft tissue healing, in which the use of solid/clot and liquid/injectable was reported. This was followed by implant stability and bone regeneration, where 4 different CPs were implemented.
Discussion
In recent years, the use of PRF in dentistry and maxillofacial surgery has broaden. The constant introduction of new protocols, i.e., most recently horizontal centrifugation, and without enough time for the new knowledge to settle in, omission to report the CPs correctly continue to be a burden for the scientific community. During the review, some difficulties were encountered in order to obtain all the information related to the protocols. A handmade search was necessary in order to complete the information needed to describe the protocols available. Additionally, when necessary, the corresponding authors were contacted to request additional information. Nevertheless, the limited number of data sources and the manually checked journals can be considered a limitation of the study. The results of this work showed a great heterogeneity in the reported protocols, patients characteristics and pathologies treated. Although there are in vitro and in vivo studies demonstrating the superiority of PRF CPs over others, concerning cellular proliferation, osteogenic differentiation and vascular formation, this research did not find such a clinical study (145,146). Furthermore, studies with a high scientific evidence (i.e., clinical trials) comparing the superiority of one single CP to treat one specific pathology were not found, and therefore recommendations cannot be made in this regard. Further studies are needed to understand whether other components in this cell concentrate, not affected or slightly affected by the CP, are playing a pivotal role in its tissue regeneration capacity. For this reason, this work introduces the AR2T3 acronym. The acronym includes all principles that affect PRF through centrifugation and can serve as an instrument to increase the understanding of PRF and its reproducibility in future studies.
The AR2T3 principles to report the centrifugation of Platelet concentrates
Angle/Radius maximum (r-max)
The angulations in which the tube is centrifuged and the r-max significantly affects the g-force applied to the tube (Figure 2). For example, based on the mathematical formulas stated in Table 9 (147), a slight increase in the r-max will significantly increase the g-force, ultimately affecting the final PRF generated in terms of cell content, size of clot, the distribution of the cells within the clot and indeed the entire morphology of the PRF clot. This was assessed by Miron et al. who recently described that horizontal centrifugation yielded PRF with more accumulations of leukocytes and platelets in comparison to the PRF produced via the fixed-angled technique (144).

Table 9
RCF (×g) = relative centrifugation force, RPM = revolutions per minute. rmax = radius of rotor measured from the axis of the rotor to the base of the tube expressed in cm.
As shown by the results of this review, the majority of the studies failed to report the angulation of the tubes and the radius of the rotor (Tables 3-7). It is therefore essential that future research in this field clearly states the tube angulation and the radius of the rotor of the centrifuge to ensure uniformity of reports and reproducibility of the protocols by other authors.
RCF (g)
For a PRF study to be reproducible, it needs to include the specifications of a precise centrifugation conditions in terms of RCF expressed in units of gravity (×g) (148) (Figure 3). A higher g-force exerted over the blood components, increases its sedimentation speed in the blood plasma according to each blood component’s mass (e.g., white blood cells and red blood cells). The fragmentation of blood gives as a result a PRF clot. If the RCF changes, the fragmentation ratio of all blood components changes and consequently the content of PRF. A clinician can always control the RPM in the available centrifuges. But the table centrifuge machines have normally a fixed radius, as observed in many of the papers reviewed. The radius and the angle can vary in different brands of commercially available centrifuges (Table 8). If a clinician tries to reproduce one of the protocols collected in this review with a centrifuge machine where the radius and angle at which the tubes spin differ from the original article, the content of the PRF clot will differ.

It is also important to note that the g-force should always be calculated at the RCF-max as this can be easily standardized and reproduced as opposed to calculating it at RCF-clot which can alter depending on many factors as elucidated by Miron et al. (149) and Ghanaati et al. (150) in their recent reviews. They were able to point out that the RPM, time, angle of the tube and the rotor size affect the position of the clot and therefore a change in these variables will lead to changes in the g-force. Additionally, they opined that the differences in the packed cell volume (PCV) levels of individual subjects could also lead to varying positions of clot formation along the tube during PRF collection. Therefore, to avoid all these errors in wrongly reporting the RCF, it is safest to report the g-force at the RCF-max i.e. at the end of the tube, which is a constant measurement, differently to the RCF-clot. Putting all these factors into consideration will ensure future reproducibility of research methodologies.
Time of centrifugation (T)
The time of centrifugation determines the distance the cells travel within the tube. The development from advanced PRF (A-PRF) to advanced PRF-plus (A-PRF+) is an example of the principle. The A-PRF and A-PRF+ protocols have the same RPM and RCF using an 11 cm radius rotor with a fixed-angel, but the time was reduced, causing a greater concentration of white blood cells to remain higher in the PRF clot (Figure 4) (18). Increasing the centrifugation time and speed leads to the formation of a larger size PRF clot. Nevertheless, the clot formed in this instance has been shown to contain less cells and growth factors (40). By reducing the RCF, a higher content of cells was found in the solid and liquid versions of PRF. The description of this natural behavior was called the Low Speed centrifugation concept (LSCC). A lower RCF slows the sedimentation speed and might result in a smaller PRF clot and a reduced fraction of liquid/injectable PRF. On the other hand, a lower RCF produces clots with evenly distributed platelets and a higher concentration of growth factors. This has already been described by previous studies who postulated that reducing the RCF and thereby using the so-called LSCC, they were able to selectively increase the accumulation of leukocytes, platelets and growth factors within the injectable PRF (9,151). This approach, according to the studies cited above, may improve the regenerative potential of PRF.

Tubes size and characteristics
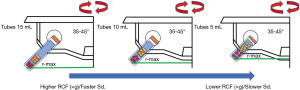
The search showed three different types of tubes to produce solid/clot PRF and one to produce liquid/injectable PRF (94,97,115,133) (Figure 5). A recent study showed that the characteristics of the tubes determine the quality of the solid/clot of PRF independently of the protocol used (43). References about the use of a titanium tube were found in the literature. It is hypothesize that the titanium tubes may be more effective in activating platelets and reducing the risk of silicosis caused by silica-coated tubes (152). In order to confirm this hypothesis further studies are necessary. In the case of the liquid/injectable PRF, the community seems to have reached a consensus on the use of plastic tubes. Furthermore, during the review it was observed that a variety of tube sizes ranging from 5 to 15 mL are being used. The tube’s size determines the final r-max, which is measured by measuring the distance from the rotational axis of the centrifuge to the bottom of the tube. In this sense, a shorter tube results in a shorter r-max and lower RCF (×g) (Figure 5). Up to date, systematic studies characterizing PRF have been performed using mainly 9 or 10 mL tubes. However, in smaller tubes, cells require less time to sediment at the bottom of the tube and the cell content is altered.
Taken all into consideration, to systematically conduct research on platelets concentrate with reproducible results, the angle of the rotor, r-max, RPM (×g), time of centrifugation, size and characteristics of the tubes need to be specified (AR2T3). Modification of these 6 components could give innumerous combinations of platelet concentrates to researchers with the option to develop direct therapies toward specific pathologies. Furthermore, the use of the acronym will allow the elaboration of meta-analyses and guidelines that are not yet available due to the heterogeneity of the data. Moreover, it will help unmask whether or not there is any significance in further fine-tuning the platelet concentrates, or if there are variables that are not yet taken into consideration. Noteworthy, the protocols of centrifugation reported in RPM are only reproducible if the clinician is using exactly the same centrifuge machine reported by the author of the protocol. On the contrary, protocols reported on g-force can be adjusted to any type of centrifuge. This applies for vertical and horizontal centrifugation.
Three mathematical equations can be found in the literature that allow clinicians to calculate missing data from previous articles and achieve reproducible PRF results in any centrifuge machine available to them (see Table 9). Finally, we provide a template to report protocols of platelet concentrates according to the AR2T3 principles: ‘‘PRF was centrifuged using a table-top centrifuge (Name of the centrifuge machine; angle X°, X cm radius-max) following a standardized protocol described by X et al. (X rpm, X g, X min). Blood was withdrawn according to the best practices in phlebotomy into X-mL sterile (characteristic of the tube; plastic, glass or titanium) tubes (Name of the manufacture). This protocol was reported following the AR2T3 acronym’’ (see example in Table 10).
Table 10
| Low RCF | Middle RCF | High RCF | |
|---|---|---|---|
| AR2T3 (solid) | |||
| A | 41.3° | 41.3° | 41.3° |
| R | 11 cm | 11 cm | 11 cm |
| R | 44 g | 177 | 710 |
| T | 8 min | 8 min | 8 min |
| T | 10 mL | 10 mL | 10 mL |
| T | Glass | Glass | Glass |
| AR2T3 (liquid) | |||
| A | 41.3° | 41.3° | 41.3° |
| R | 11 cm | 11 cm | 11 cm |
| R | 44 g | 177 | 710 |
| T | 8 min | 8 min | 8 min |
| T | 10 mL | 10 mL | 10 mL |
| T | Plastic | Plastic | Plastic |
Protocol: the “Low Speed Centrifugation Concept (LSCC)”; Centrifuge: PRF DUO Process. PRF, platelet-rich fibrin; RCF, relative centrifugation force.
Conclusions
The centrifugation protocol (CP) is one of the most important steps in the generation of PRF as it affects the final characteristics. The present review analyzed CPs used in oral and maxillofacial surgery. The findings indicate that many authors and researchers gave incomplete details regarding the centrifugation machines and the protocols used in obtaining their PRF samples. In fact, only a few of the reviewed articles gave enough information that could make their works reproducible by other researchers. This paucity of detailed reporting has led to various errors in methodologies with some variables like g-force being wrongly calculated and applied, thereby leading to misinformation as to the correct g-force applied during centrifugation. This review has therefore exposed three mathematical equations that can be used by PRF researchers and clinicians in the future to calculate all the missing variables in any previous incomplete research methodology thereby ensuring accurate reporting and duplication of the methods. Additionally, it is here proposed the AR2T3 acronym as a guide for all authors in the field of platelet concentrates to ensure uniformity in reporting of the protocols. Adherence to this recommended guideline will ensure uniformity of the CP and harmonization in the field.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The author has completed the PRISMA reporting checklist. Available at https://fomm.amegroups.com/article/view/10.21037/fomm-21-40/rc
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://fomm.amegroups.com/article/view/10.21037/fomm-21-40/coif). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Centrifugation. In: Laboratory Techniques in Biochemistry and Molecular Biology. 1988:18-69.
- Kellet BE, Han B, Dandy DS, et al. Modeling centrifugal cell washers using computational fluid dynamics. Artif Organs 2004;28:1026-34. [Crossref] [PubMed]
- Holtzhauer M. Basic Manuals for the Biochemical Lab. Heidelberg: Springer-Verlag Berlin, 2006.
- Mussano F, Genova T, Munaron L, et al. Cytokine, chemokine, and growth factor profile of platelet-rich plasma. Platelets 2016;27:467-71. [Crossref] [PubMed]
- Miron RJ, Dham A, Dham U, et al. The effect of age, gender, and time between blood draw and start of centrifugation on the size outcomes of platelet-rich fibrin (PRF) membranes. Clin Oral Investig 2019;23:2179-85. [Crossref] [PubMed]
- Miron RJ, Xu H, Chai J, et al. Comparison of platelet-rich fibrin (PRF) produced using 3 commercially available centrifuges at both high (~ 700 g) and low (~ 200 g) relative centrifugation forces. Clin Oral Investig 2020;24:1171-82. [Crossref] [PubMed]
- Ghanaati S, Herrera-Vizcaino C, Al-Maawi S, et al. Fifteen Years of Platelet Rich Fibrin in Dentistry and Oromaxillofacial Surgery: How High is the Level of Scientific Evidence? J Oral Implantol 2018;44:471-92. [Crossref] [PubMed]
- Albilia J. Liquid platelet-rich fibrin injections as a treatment adjunct for painful temporomandibular joints: preliminary results. Cranio 2020;38:292-304. [Crossref] [PubMed]
- Kubesch A, Barbeck M, Al-Maawi S, et al. A low-speed centrifugation concept leads to cell accumulation and vascularization of solid platelet-rich fibrin: an experimental study in vivo. Platelets 2019;30:329-40. [Crossref] [PubMed]
- Fernández-Delgado N, Hernández-Ramírez P, Forrellat-Barrios M. Pletelet functional spectrum: from hemostasis to regenerative medicine. Rev Cuba Hematol Inmunol y Hemoter 2012;28:200-16.
- Vogler EA, Siedlecki CA. Contact activation of blood-plasma coagulation. Biomaterials 2009;30:1857-69. [Crossref] [PubMed]
- Kratz A, Stanganelli N, Van Cott EM. A comparison of glass and plastic blood collection tubes for routine and specialized coagulation assays: a comprehensive study. Arch Pathol Lab Med 2006;130:39-44. [Crossref] [PubMed]
- Osterud B, Brox JH. The clotting time of whole blood in plastic tubes: the influence of exercise, prostacyclin and acetylsalicylic acid. Thromb Res 1983;29:425-35. [Crossref] [PubMed]
- He L, Lin Y, Hu X, et al. A comparative study of platelet-rich fibrin (PRF) and platelet-rich plasma (PRP) on the effect of proliferation and differentiation of rat osteoblasts in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009;108:707-13. [Crossref] [PubMed]
- Herrera-Vizcaino C, Albilia JB. Temporomandibular joint biosupplementation using platelet concentrates: a narrative review. Front Oral Maxillofac Med 2021; [Crossref]
- Lacy P. Mechanisms of degranulation in neutrophils. Allergy Asthma Clin Immunol 2006;2:98-108. [Crossref] [PubMed]
- Leliefeld PH, Wessels CM, Leenen LP, et al. The role of neutrophils in immune dysfunction during severe inflammation. Crit Care 2016;20:73. [Crossref] [PubMed]
- El Bagdadi K, Kubesch A, Yu X, et al. Reduction of relative centrifugal forces increases growth factor release within solid platelet-rich-fibrin (PRF)-based matrices: a proof of concept of LSCC (low speed centrifugation concept). Eur J Trauma Emerg Surg 2019;45:467-79. [Crossref] [PubMed]
- Neumann FJ, Marx N, Gawaz M, et al. Induction of cytokine expression in leukocytes by binding of thrombin-stimulated platelets. Circulation 1997;95:2387-94. [Internet].
- Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part III: leucocyte activation: a new feature for platelet concentrates? Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;101:e51-5. [Crossref] [PubMed]
- Dohan Ehrenfest DM, Pinto NR, Pereda A, et al. The impact of the centrifuge characteristics and centrifugation protocols on the cells, growth factors, and fibrin architecture of a leukocyte- and platelet-rich fibrin (L-PRF) clot and membrane. Platelets 2018;29:171-84. [Crossref] [PubMed]
- Egger M, Smith GD, Altman DG. Systematic Reviews in Health Care: Meta-Analysis in Context. BMJ Books, editor. NJ, USA: John Wiley & Sons, 2001.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [Crossref] [PubMed]
- Moussa M, El-Dahab OA, El Nahass H. Anterior Maxilla Augmentation Using Palatal Bone Block with Platelet-Rich Fibrin: A Controlled Trial. Int J Oral Maxillofac Implants 2016;31:708-15. [Crossref] [PubMed]
- Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;101:e37-44. [Crossref] [PubMed]
- Aroca S, Keglevich T, Barbieri B, et al. Clinical evaluation of a modified coronally advanced flap alone or in combination with a platelet-rich fibrin membrane for the treatment of adjacent multiple gingival recessions: a 6-month study. J Periodontol 2009;80:244-52. [Crossref] [PubMed]
- Sharma A, Pradeep AR. Treatment of 3-wall intrabony defects in patients with chronic periodontitis with autologous platelet-rich fibrin: a randomized controlled clinical trial. J Periodontol 2011;82:1705-12. [Crossref] [PubMed]
- Sharma A, Pradeep AR. Autologous platelet-rich fibrin in the treatment of mandibular degree II furcation defects: a randomized clinical trial. J Periodontol 2011;82:1396-403. [Crossref] [PubMed]
- Simonpieri A, Choukroun J, Del Corso M, et al. Simultaneous sinus-lift and implantation using microthreaded implants and leukocyte- and platelet-rich fibrin as sole grafting material: a six-year experience. Implant Dent 2011;20:2-12. [Crossref] [PubMed]
- Tatullo M, Marrelli M, Cassetta M, et al. Platelet Rich Fibrin (P.R.F.) in reconstructive surgery of atrophied maxillary bones: clinical and histological evaluations. Int J Med Sci 2012;9:872-80. [Crossref] [PubMed]
- Jankovic S, Aleksic Z, Klokkevold P, et al. Use of platelet-rich fibrin membrane following treatment of gingival recession: a randomized clinical trial. Int J Periodontics Restorative Dent 2012;32:e41-50.
- Singh S, Singh A, Singh S, et al. Application of PRF in surgical management of periapical lesions. Natl J Maxillofac Surg 2013;4:94-9. [Crossref] [PubMed]
- Bajaj P, Pradeep AR, Agarwal E, et al. Comparative evaluation of autologous platelet-rich fibrin and platelet-rich plasma in the treatment of mandibular degree II furcation defects: a randomized controlled clinical trial. J Periodontal Res 2013;48:573-81. [Crossref] [PubMed]
- Bansal C, Bharti V. Evaluation of efficacy of autologous platelet-rich fibrin with demineralized-freeze dried bone allograft in the treatment of periodontal intrabony defects. J Indian Soc Periodontol 2013;17:361-6. [Crossref] [PubMed]
- Suttapreyasri S, Leepong N. Influence of platelet-rich fibrin on alveolar ridge preservation. J Craniofac Surg 2013;24:1088-94. [Crossref] [PubMed]
- Geeta IB, Galagali G, Kulkarni S, et al. A natural meliorate: revolutionary tissue engineering in endodontics. J Clin Diagn Res 2013;7:2644-6. [Crossref] [PubMed]
- Soydan SS, Uckan S. Management of bisphosphonate-related osteonecrosis of the jaw with a platelet-rich fibrin membrane: technical report. J Oral Maxillofac Surg 2014;72:322-6. [Crossref] [PubMed]
- Joseph V R, Sam G, Amol NV. Clinical evaluation of autologous platelet rich fibrin in horizontal alveolar bony defects. J Clin Diagn Res 2014;8:ZC43-7. [Crossref] [PubMed]
- Yelamali T, Saikrishna D. Role of platelet rich fibrin and platelet rich plasma in wound healing of extracted third molar sockets: a comparative study. J Maxillofac Oral Surg 2015;14:410-6. [Crossref] [PubMed]
- Kumar N, Prasad K, Ramanujam L, et al. Evaluation of treatment outcome after impacted mandibular third molar surgery with the use of autologous platelet-rich fibrin: a randomized controlled clinical study. J Oral Maxillofac Surg 2015;73:1042-9. [Crossref] [PubMed]
- Inchingolo F, Ballini A, Mura SA, et al. Use of platelet rich fibrin and Bio-OSS/SINT-Oss for implant-prosthetic rehabilitation in maxillary atrophy with sinus pathology: A 48-month follow-up. Eur J Inflamm 2015;13:58-65.
- Dhiman M, Kumar S, Duhan J, et al. Effect of Platelet-rich Fibrin on Healing of Apicomarginal Defects: A Randomized Controlled Trial. J Endod 2015;41:985-91. [Crossref] [PubMed]
- Hamzacebi B, Oduncuoglu B, Alaaddinoglu EE. Treatment of Peri-implant Bone Defects with Platelet-Rich Fibrin. Int J Periodontics Restorative Dent 2015;35:415-22. [Crossref] [PubMed]
- Boora P, Rathee M, Bhoria M. Effect of Platelet Rich Fibrin (PRF) on Peri-implant Soft Tissue and Crestal Bone in One-Stage Implant Placement: A Randomized Controlled Trial. J Clin Diagn Res 2015;9:ZC18-21. [Crossref] [PubMed]
- Shawky H, Seifeldin SA. Does Platelet-Rich Fibrin Enhance Bone Quality and Quantity of Alveolar Cleft Reconstruction? Cleft Palate Craniofac J 2016;53:597-606. [Crossref] [PubMed]
- Ajwani H, Shetty S, Gopalakrishnan D, et al. Comparative evaluation of platelet-rich fibrin biomaterial and open flap debridement in the treatment of two and three wall intrabony defects. J Int Oral Health 2015;7:32-7.
- Mathur A, Bains VK, Gupta V, et al. Evaluation of intrabony defects treated with platelet-rich fibrin or autogenous bone graft: A comparative analysis. Eur J Dent 2015;9:100-8. [Crossref] [PubMed]
- Pradeep AR, Nagpal K, Karvekar S, et al. Platelet-rich fibrin with 1% metformin for the treatment of intrabony defects in chronic periodontitis: a randomized controlled clinical trial. J Periodontol 2015;86:729-37. [Crossref] [PubMed]
- Sandhu GK, Khinda PK, Gill AS, et al. Surgical re-entry evaluation of regenerative efficacy of bioactive Gengigel(®) and platelet-rich fibrin in the treatment of grade II furcation: A novel approach. Contemp Clin Dent 2015;6:570-3. [Crossref] [PubMed]
- Pradeep AR, Karvekar S, Nagpal K, et al. Rosuvastatin 1.2 mg In Situ Gel Combined With 1:1 Mixture of Autologous Platelet-Rich Fibrin and Porous Hydroxyapatite Bone Graft in Surgical Treatment of Mandibular Class II Furcation Defects: A Randomized Clinical Control Trial. J Periodontol 2016;87:5-13. [Crossref] [PubMed]
- Doiphode AM, Hegde P, Mahindra U, et al. Evaluation of the efficacy of platelet-rich plasma and platelet-rich fibrin in alveolar defects after removal of impacted bilateral mandibular third molars. J Int Soc Prev Community Dent 2016;6:S47-52. [Crossref] [PubMed]
- Munoz F, Jiménez C, Espinoza D, et al. Use of leukocyte and platelet-rich fibrin (L-PRF) in periodontally accelerated osteogenic orthodontics (PAOO): Clinical effects on edema and pain. J Clin Exp Dent 2016;8:e119-24. [Crossref] [PubMed]
- Kanayama T, Horii K, Senga Y, et al. Crestal Approach to Sinus Floor Elevation for Atrophic Maxilla Using Platelet-Rich Fibrin as the Only Grafting Material: A 1-Year Prospective Study. Implant Dent 2016;25:32-8. [Crossref] [PubMed]
- Kumar YR, Mohanty S, Verma M, et al. Platelet-rich fibrin: the benefits. Br J Oral Maxillofac Surg 2016;54:57-61. [Crossref] [PubMed]
- Subash D, Shoba K, Aman S, et al. Revitalization of an Immature Permanent Mandibular Molar with a Necrotic Pulp Using Platelet-Rich Fibrin: A Case Report. J Clin Diagn Res 2016;10:ZD21-3. [Crossref] [PubMed]
- Asaka T, Ohga N, Yamazaki Y, et al. Platelet-rich fibrin may reduce the risk of delayed recovery in tooth-extracted patients undergoing oral bisphosphonate therapy: a trial study. Clin Oral Investig 2017;21:2165-72. [Crossref] [PubMed]
- Park JH, Kim JW, Kim SJ. Does the Addition of Bone Morphogenetic Protein 2 to Platelet-Rich Fibrin Improve Healing After Treatment for Medication-Related Osteonecrosis of the Jaw? J Oral Maxillofac Surg 2017;75:1176-84. [Crossref] [PubMed]
- Kanoriya D, Pradeep AR, Garg V, et al. Mandibular Degree II Furcation Defects Treatment With Platelet-Rich Fibrin and 1% Alendronate Gel Combination: A Randomized Controlled Clinical Trial. J Periodontol 2017;88:250-8. [Crossref] [PubMed]
- Varghese MP, Manuel S, Kumar L K S. Potential for Osseous Regeneration of Platelet-Rich Fibrin-A Comparative Study in Mandibular Third Molar Impaction Sockets. J Oral Maxillofac Surg 2017;75:1322-9. [Crossref] [PubMed]
- Bilginaylar K. Uncommon Odontogenic Orocutaneous Fistula of the Jaw Treated with Platelet-Rich Fibrin. Case Rep Dent 2017;2017:7174217. [Crossref] [PubMed]
- Zhang J, Qi X, Luo X, et al. Clinical and immunohistochemical performance of lyophilized platelet-rich fibrin (Ly-PRF) on tissue regeneration. Clin Implant Dent Relat Res 2017;19:466-77. [Crossref] [PubMed]
- Gülşen U, Şentürk MF. Effect of platelet rich fibrin on edema and pain following third molar surgery: a split mouth control study. BMC Oral Health 2017;17:79. [Crossref] [PubMed]
- Goel S, Nawal RR, Talwar S. Management of Dens Invaginatus Type II Associated with Immature Apex and Large Periradicular Lesion Using Platelet-rich Fibrin and Biodentine. J Endod 2017;43:1750-5. [Crossref] [PubMed]
- Cömert Kılıç S, Güngörmüş M, Parlak SN. Histologic and histomorphometric assessment of sinus-floor augmentation with beta-tricalcium phosphate alone or in combination with pure-platelet-rich plasma or platelet-rich fibrin: A randomized clinical trial. Clin Implant Dent Relat Res 2017;19:959-67.
- Pripatnanont P, Thanakone P, Leepong N. Dimensional Change and Microstructure of Intraoral Bone Block Grafts Covered with Platelet-Rich Fibrin and a Barrier Membrane in Ridge Augmentation: A Pilot Investigation. Int J Periodontics Restorative Dent 2017;37:693-703. [Crossref] [PubMed]
- Bajaj P, Agarwal E, Rao NS, et al. Autologous Platelet-Rich Fibrin in the Treatment of 3-Wall Intrabony Defects in Aggressive Periodontitis: A Randomized Controlled Clinical Trial. J Periodontol 2017;88:1186-91. [Crossref] [PubMed]
- Patel GK, Gaekwad SS, Gujjari SK, et al. Platelet-Rich Fibrin in Regeneration of Intrabony Defects: A Randomized Controlled Trial. J Periodontol 2017;88:1192-9. [Crossref] [PubMed]
- Sharma S, Sharma V, Passi D, et al. Large Periapical or Cystic Lesions in Association with Roots Having Open Apices Managed Nonsurgically Using 1-step Apexification Based on Platelet-rich Fibrin Matrix and Biodentine Apical Barrier: A Case Series. J Endod 2018;44:179-85. [Crossref] [PubMed]
- Afat İM, Akdoğan ET, Gönül O. Effects of Leukocyte- and Platelet-Rich Fibrin Alone and Combined With Hyaluronic Acid on Pain, Edema, and Trismus After Surgical Extraction of Impacted Mandibular Third Molars. J Oral Maxillofac Surg 2018;76:926-32. [Crossref] [PubMed]
- Eshghpour M, Danaeifar N, Kermani H, et al. Does Intra-Alveolar Application of Chlorhexidine Gel in Combination With Platelet-Rich Fibrin Have an Advantage Over Application of Platelet-Rich Fibrin in Decreasing Alveolar Osteitis After Mandibular Third Molar Surgery? A Double-Blinded Randomized Clinical Trial. J Oral Maxillofac Surg 2018;76:939.e1-7. [Crossref] [PubMed]
- Asmael HM, Jamil FA, Hasan AM. Novel Application of Platelet-Rich Fibrin as a Wound Healing Enhancement in Extraction Sockets of Patients Who Smoke. J Craniofac Surg 2018;29:e794-7. [Crossref] [PubMed]
- Bahammam MA. Effect of platelet-rich fibrin palatal bandage on pain scores and wound healing after free gingival graft: a randomized controlled clinical trial. Clin Oral Investig 2018;22:3179-88. [Crossref] [PubMed]
- Pichotano EC, de Molon RS, Freitas de Paula LG, et al. Early Placement of Dental Implants in Maxillary Sinus Grafted With Leukocyte and Platelet-Rich Fibrin and Deproteinized Bovine Bone Mineral. J Oral Implantol 2018;44:199-206. [Crossref] [PubMed]
- Pinto GDDS, Pigossi SC, Pessoa T, et al. Successful Use of Leukocyte Platelet-Rich Fibrin in the Healing of Sinus Membrane Perforation: A Case Report. Implant Dent 2018;27:375-80. [Crossref] [PubMed]
- Zhou J, Li X, Sun X, et al. Bone regeneration around immediate placed implant of molar teeth with autologous platelet-rich fibrin: Two case reports. Medicine (Baltimore) 2018;97:e13058. [Crossref] [PubMed]
- Unsal H. H Erbasar GN. Evaluation of the Effect of Platelet-Rich Fibrin on the Alveolar Osteitis Incidence and Periodontal Probing Depth after Extracting Partially Erupted Mandibular Third Molars Extraction. Niger J Clin Pract 2018;21:201-5. [Crossref] [PubMed]
- Santhakumar M, Yayathi S, Retnakumari N. A clinicoradiographic comparison of the effects of platelet-rich fibrin gel and platelet-rich fibrin membrane as scaffolds in the apexification treatment of young permanent teeth. J Indian Soc Pedod Prev Dent 2018;36:65-70. [Crossref] [PubMed]
- Nageh M, Ahmed GM, El-Baz AA. Assessment of Regaining Pulp Sensibility in Mature Necrotic Teeth Using a Modified Revascularization Technique with Platelet-rich Fibrin: A Clinical Study. J Endod 2018;44:1526-33. [Crossref] [PubMed]
- Mahajan M, Gupta MK, Bande C, et al. Comparative Evaluation of Healing Pattern After Surgical Excision of Oral Mucosal Lesions by Using Platelet-Rich Fibrin (PRF) Membrane and Collagen Membrane as Grafting Materials-A Randomized Clinical Trial. J Oral Maxillofac Surg 2018;76:1469.e1-9. [Crossref] [PubMed]
- Bilginaylar K. Comparison of the Clinical Outcomes of Buccal Advancement Flap Versus Platelet-Rich Fibrin Application for the Immediate Closure of Acute Oroantral Communications. J Craniofac Surg 2019;30:e45-9. [Crossref] [PubMed]
- Lv H, Chen Y, Cai Z, et al. The efficacy of platelet-rich fibrin as a scaffold in regenerative endodontic treatment: a retrospective controlled cohort study. BMC Oral Health 2018;18:139. [Crossref] [PubMed]
- Mazzone N, Mici E, Calvo A, et al. Preliminary Results of Bone Regeneration in Oromaxillomandibular Surgery Using Synthetic Granular Graft. Biomed Res Int 2018;2018:8503427. [Crossref] [PubMed]
- Caymaz MG, Uyanik LO. Comparison of the effect of advanced platelet-rich fibrin and leukocyte- and platelet-rich fibrin on outcomes after removal of impacted mandibular third molar: A randomized split-mouth study. Niger J Clin Pract 2019;22:546-52. [Crossref] [PubMed]
- Diana C, Mohanty S, Chaudhary Z, et al. Does platelet-rich fibrin have a role in osseointegration of immediate implants? A randomized, single-blind, controlled clinical trial. Int J Oral Maxillofac Surg 2018;47:1178-88. [Crossref] [PubMed]
- Chang YC, Zhao JH. Effects of platelet-rich fibrin on human periodontal ligament fibroblasts and application for periodontal infrabony defects. Aust Dent J 2011;56:365-71. [Crossref] [PubMed]
- Bains R, Bains VK, Loomba K, et al. Management of pulpal floor perforation and grade II Furcation involvement using mineral trioxide aggregate and platelet rich fibrin: A clinical report. Contemp Clin Dent 2012;3:S223-7. [Crossref] [PubMed]
- Dar M, Hakim T, Shah A, et al. Use of autologous platelet-rich fibrin in osseous regeneration after cystic enucleation: A clinical study. J Oral Biol Craniofac Res 2016;6:S29-32. [Crossref] [PubMed]
- Khan ZA, Jhingran R, Bains VK, et al. Evaluation of peri-implant tissues around nanopore surface implants with or without platelet rich fibrin: a clinico-radiographic study. Biomed Mater 2018;13:025002. [Crossref] [PubMed]
- Thorat M, Baghele ON. S RP. Adjunctive Effect of Autologous Platelet-Rich Fibrin in the Treatment of Intrabony Defects in Localized Aggressive Periodontitis Patients: A Randomized Controlled Split-Mouth Clinical Trial. Int J Periodontics Restorative Dent 2017;37:e302-9. [Crossref] [PubMed]
- Ragab RA, Lattif AEAE, Dokky NAEWE. Comparative Study between Revitalization of Necrotic Immature Permanent Anterior Teeth with and without Platelet Rich Fibrin: A Randomized Controlled Trial. J Clin Pediatr Dent 2019;43:78-85. [Crossref] [PubMed]
- Kizildağ A, Çiçek Y, Arabaci T, et al. The effect of leukocyte-platelet-rich fibrin on bone morphogenetic protein-2 and insulin-like growth factor-1 levels in patients with chronic periodontitis: a randomized split mouth clinical trail. Growth Factors 2018;36:239-45. [Crossref] [PubMed]
- Al-Ahmady HH, Abd Elazeem AF, Bellah Ahmed NE, et al. Combining autologous bone marrow mononuclear cells seeded on collagen sponge with Nano Hydroxyapatite, and platelet-rich fibrin: Reporting a novel strategy for alveolar cleft bone regeneration. J Craniomaxillofac Surg 2018;46:1593-600. [Crossref] [PubMed]
- Cortese A, Pantaleo G, Borri A, et al. Platelet-rich fibrin (PRF) in implant dentistry in combination with new bone regenerative technique in elderly patients. Int J Surg Case Rep 2016;28:52-6. [Crossref] [PubMed]
- Ustaoğlu G, Ercan E, Tunali M. The role of titanium-prepared platelet-rich fibrin in palatal mucosal wound healing and histoconduction. Acta Odontol Scand 2016;74:558-64. [Crossref] [PubMed]
- Arabacı T, Kose O, Albayrak M, et al. Advantages of Autologous Platelet-Rich Fibrin Membrane on Gingival Crevicular Fluid Growth Factor Levels and Periodontal Healing: A Randomized Split-Mouth Clinical Study. J Periodontol 2017;88:771-7. [Crossref] [PubMed]
- Tabrizi R, Arabion H, Karagah T. Does platelet-rich fibrin increase the stability of implants in the posterior of the maxilla? A split-mouth randomized clinical trial. Int J Oral Maxillofac Surg 2018;47:672-5. [Crossref] [PubMed]
- Pirebas HG, Hendek MK, Kisa U, et al. Effect of titanium-prepared platelet-rich fibrin treatment on the angiogenic biomarkers in gingival crevicular fluid in infrabony defects of patients with chronic periodontitis: A randomized controlled clinical trial. Niger J Clin Pract 2018;21:69-75. [Crossref] [PubMed]
- Daugela P, Grimuta V, Sakavicius D, et al. Influence of leukocyte- and platelet-rich fibrin (L-PRF) on the outcomes of impacted mandibular third molar removal surgery: A split-mouth randomized clinical trial. Quintessence Int 2018;49:377-88. [Crossref] [PubMed]
- Sammartino G, Dohan Ehrenfest DM, Carile F, et al. Prevention of hemorrhagic complications after dental extractions into open heart surgery patients under anticoagulant therapy: the use of leukocyte- and platelet-rich fibrin. J Oral Implantol 2011;37:681-90. [Crossref] [PubMed]
- Ruga E, Gallesio C, Boffano P. Platelet-rich fibrin and piezoelectric surgery: a safe technique for the prevention of periodontal complications in third molar surgery. J Craniofac Surg 2011;22:1951-5. [Crossref] [PubMed]
- Marenzi G, Riccitiello F, Tia M, et al. Influence of Leukocyte- and Platelet-Rich Fibrin (L-PRF) in the Healing of Simple Postextraction Sockets: A Split-Mouth Study. Biomed Res Int 2015;2015:369273. [Crossref] [PubMed]
- Öncü E, Alaaddinoğlu EE. The effect of platelet-rich fibrin on implant stability. Int J Oral Maxillofac Implants 2015;30:578-82. [Crossref] [PubMed]
- Kotsakis GA, Boufidou F, Hinrichs JE, et al. Extraction Socket Management Utilizing Platelet Rich Fibrin: A Proof-of-Principle Study of the "Accelerated-Early Implant Placement" Concept. J Oral Implantol 2016;42:164-8. [Crossref] [PubMed]
- Bakhtiar H, Esmaeili S, Fakhr Tabatabayi S, et al. Second-generation Platelet Concentrate (Platelet-rich Fibrin) as a Scaffold in Regenerative Endodontics: A Case Series. J Endod 2017;43:401-8. [Crossref] [PubMed]
- Kapustecki M, Niedzielska I, Borgiel-Marek H, et al. Alternative method to treat oroantral communication and fistula with autogenous bone graft and platelet rich fibrin. Med Oral Patol Oral Cir Bucal 2016;21:e608-13. [Crossref] [PubMed]
- Anwandter A, Bohmann S, Nally M, et al. Dimensional changes of the post extraction alveolar ridge, preserved with Leukocyte- and Platelet Rich Fibrin: A clinical pilot study. J Dent 2016;52:23-9. [Crossref] [PubMed]
- Gönen ZB, Yılmaz Asan C. Treatment of bisphosphonate-related osteonecrosis of the jaw using platelet-rich fibrin. Cranio 2017;35:332-6. [Crossref] [PubMed]
- Gurler G, Delilbasi C. Effects of leukocyte-platelet rich fibrin on postoperative complications of direct sinus lifting. Minerva Stomatol 2016;65:207-12.
- Temmerman A, Vandessel J, Castro A, et al. The use of leucocyte and platelet-rich fibrin in socket management and ridge preservation: a split-mouth, randomized, controlled clinical trial. J Clin Periodontol 2016;43:990-9. [Crossref] [PubMed]
- Öncü E, Erbeyoğlu AA. Enhancement of Immediate Implant Stability and Recovery Using Platelet-Rich Fibrin. Int J Periodontics Restorative Dent 2019;39:e58-e63. [Crossref] [PubMed]
- Sezgin Y, Uraz A, Taner IL, et al. Effects of platelet-rich fibrin on healing of intra-bony defects treated with anorganic bovine bone mineral. Braz Oral Res 2017;31:e15. [Crossref] [PubMed]
- Ozcan M, Ucak O, Alkaya B, et al. Effects of Platelet-Rich Fibrin on Palatal Wound Healing After Free Gingival Graft Harvesting: A Comparative Randomized Controlled Clinical Trial. Int J Periodontics Restorative Dent 2017;37:e270-8. [Crossref] [PubMed]
- Patidar S, Kalra N, Khatri A, et al. Clinical and radiographic comparison of platelet-rich fibrin and mineral trioxide aggregate as pulpotomy agents in primary molars. J Indian Soc Pedod Prev Dent 2017;35:367-73. [Crossref] [PubMed]
- Pinto N, Harnish A, Cabrera C, et al. An Innovative Regenerative Endodontic Procedure Using Leukocyte and Platelet-rich Fibrin Associated with Apical Surgery: A Case Report. J Endod 2017;43:1828-34. [Crossref] [PubMed]
- Uzun BC, Ercan E, Tunalı M. Effectiveness and predictability of titanium-prepared platelet-rich fibrin for the management of multiple gingival recessions. Clin Oral Investig 2018;22:1345-54. [Crossref] [PubMed]
- Shah R, Shah H, Shetty O, et al. A novel approach to treat peri implantitis with the help of PRF. Pan Afr Med J 2017;27:256. [Crossref] [PubMed]
- Betancourt P, Elgueta R, Fuentes R. Treatment of endo-periodontal lesion using leukocyte-platelet-rich fibrin. A case report. Colomb Med (Cali) 2017;48:204-7. [Crossref] [PubMed]
- Culhaoglu R, Taner L, Guler B. Evaluation of the effect of dose-dependent platelet-rich fibrin membrane on treatment of gingival recession: a randomized, controlled clinical trial. J Appl Oral Sci 2018;26:e20170278. [Crossref] [PubMed]
- Asutay F, Yolcu Ü, Geçör O, et al. An evaluation of effects of platelet-rich-fibrin on postoperative morbidities after lower third molar surgery. Niger J Clin Pract 2017;20:1531-6. [Crossref] [PubMed]
- Temmerman A, Cleeren GJ, Castro AB, et al. L-PRF for increasing the width of keratinized mucosa around implants: A split-mouth, randomized, controlled pilot clinical trial. J Periodontal Res 2018;53:793-800. [Crossref] [PubMed]
- S Medikeri R. Effect of PRF and Allograft Use on Immediate Implants at Extraction Sockets with Periapical Infection -Clinical and Cone Beam CT Findings. Bull Tokyo Dent Coll 2018;59:97-109. [Crossref] [PubMed]
- Dixit N, Lamba AK, Faraz F, et al. Root coverage by modified coronally advanced flap with and without platelet-rich fibrin: A clinical study. Indian J Dent Res 2018;29:600-4. [Crossref] [PubMed]
- Liang ZJ, Lu X, Li DQ, et al. Precise Intradermal Injection of Nanofat-Derived Stromal Cells Combined with Platelet-Rich Fibrin Improves the Efficacy of Facial Skin Rejuvenation. Cell Physiol Biochem 2018;47:316-29. [Crossref] [PubMed]
- Cortellini S, Castro AB, Temmerman A, et al. Leucocyte- and platelet-rich fibrin block for bone augmentation procedure: A proof-of-concept study. J Clin Periodontol 2018;45:624-34. [Crossref] [PubMed]
- Choukroun J, Diss A, Simonpieri A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part V: histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;101:299-303. [Crossref] [PubMed]
- Gamal AY, Abdel Ghaffar KA, Alghezwy OA. Crevicular Fluid Growth Factors Release Profile Following the Use of Platelet-Rich Fibrin and Plasma Rich Growth Factors in Treating Periodontal Intrabony Defects: A Randomized Clinical Trial. J Periodontol 2016;87:654-62. [Crossref] [PubMed]
- Lei L, Yu Y, Ke T, et al. The Application of Three-Dimensional Printing Model and Platelet-Rich Fibrin Technology in Guided Tissue Regeneration Surgery for Severe Bone Defects. J Oral Implantol 2019;45:35-43. [Crossref] [PubMed]
- Demetoglu U, Ocak H, Bilge S. Closure of Oroantral Communication With Plasma-Rich Fibrin Membrane. J Craniofac Surg 2018;29:e367-70. [Crossref] [PubMed]
- Nørholt SE, Hartlev J. Surgical treatment of osteonecrosis of the jaw with the use of platelet-rich fibrin: a prospective study of 15 patients. Int J Oral Maxillofac Surg 2016;45:1256-60. [Crossref] [PubMed]
- Chenchev IL, Ivanova VV, Neychev DZ, et al. Application of Platelet-Rich Fibrin and Injectable Platelet-Rich Fibrin in Combination of Bone Substitute Material for Alveolar Ridge Augmentation - a Case Report. Folia Med (Plovdiv) 2017;59:362-6. [Crossref] [PubMed]
- Fortunato L, Barone S, Bennardo F, et al. Management of Facial Pyoderma Gangrenosum Using Platelet-Rich Fibrin: A Technical Report. J Oral Maxillofac Surg 2018;76:1460-3. [Crossref] [PubMed]
- Lekovic V, Milinkovic I, Aleksic Z, et al. Platelet-rich fibrin and bovine porous bone mineral vs. platelet-rich fibrin in the treatment of intrabony periodontal defects. J Periodontal Res 2012;47:409-17. [Crossref] [PubMed]
- Olgun E, Ozkan SY, Atmaca HT, et al. Comparison of the clinical, radiographic, and histological effects of titanium-prepared platelet rich fibrin to allograft materials in sinus-lifting procedures. J Investig Clin Dent 2018;9:e12347. [Crossref] [PubMed]
- Choukroun J, Ghanaati S. Reduction of relative centrifugation force within injectable platelet-rich-fibrin (PRF) concentrates advances patients' own inflammatory cells, platelets and growth factors: the first introduction to the low speed centrifugation concept. Eur J Trauma Emerg Surg 2018;44:87-95. [Crossref] [PubMed]
- Meza G, Urrejola D, Saint Jean N, et al. Personalized Cell Therapy for Pulpitis Using Autologous Dental Pulp Stem Cells and Leukocyte Platelet-rich Fibrin: A Case Report. J Endod 2019;45:144-9. [Crossref] [PubMed]
- Thorat M, Pradeep AR, Pallavi B. Clinical effect of autologous platelet-rich fibrin in the treatment of intra-bony defects: a controlled clinical trial. J Clin Periodontol 2011;38:925-32. [Crossref] [PubMed]
- Peck MT, Marnewick J, Stephen L. Alveolar ridge preservation using leukocyte and platelet-rich fibrin: a report of a case. Case Rep Dent 2011;2011:345048. [Crossref] [PubMed]
- Agarwal A, Gupta ND. Platelet-rich plasma combined with decalcified freeze-dried bone allograft for the treatment of noncontained human intrabony periodontal defects: a randomized controlled split-mouth study. Int J Periodontics Restorative Dent 2014;34:705-11. [Crossref] [PubMed]
- Bolukbasi N, Ersanlı S, Keklikoglu N, et al. Sinus Augmentation With Platelet-Rich Fibrin in Combination With Bovine Bone Graft Versus Bovine Bone Graft in Combination With Collagen Membrane. J Oral Implantol 2015;41:586-95. [Crossref] [PubMed]
- Nizam N, Eren G, Akcalı A, et al. Maxillary sinus augmentation with leukocyte and platelet-rich fibrin and deproteinized bovine bone mineral: A split-mouth histological and histomorphometric study. Clin Oral Implants Res 2018;29:67-75. [Crossref] [PubMed]
- de Almeida Barros Mourão CF, Calasans-Maia MD, de Mello Machado RC, et al. The use of platelet-rich fibrin as a hemostatic material in oral soft tissues. Oral Maxillofac Surg 2018;22:329-33. [Crossref] [PubMed]
- Lorenz J, Al-Maawi S, Sader R, et al. Individualized Titanium Mesh Combined With Platelet-Rich Fibrin and Deproteinized Bovine Bone: A New Approach for Challenging Augmentation. J Oral Implantol 2018;44:345-51. [Crossref] [PubMed]
- Nacopoulos C, Gkouskou K, Karypidis D, et al. Telomere length and genetic variations affecting telomere length as biomarkers for facial regeneration with platelet-rich fibrin based on the low-speed centrifugation concept. J Cosmet Dermatol 2019;18:408-13. [Crossref] [PubMed]
- Miron RJ, Chai J, Fujioka-Kobayashi M, et al. Evaluation of 24 protocols for the production of platelet-rich fibrin. BMC Oral Health 2020;20:310. [Crossref] [PubMed]
- Herrera-Vizcaíno C, Dohle E, Al-Maawi S, et al. Platelet-rich fibrin secretome induces three dimensional angiogenic activation in vitro. Eur Cell Mater 2019;37:250-64. [Crossref] [PubMed]
- Verboket R, Herrera-Vizcaíno C, Thorwart K, et al. Influence of concentration and preparation of platelet rich fibrin on human bone marrow mononuclear cells (in vitro). Platelets 2019;30:861-70. [Crossref] [PubMed]
- Majekodunmi SO. A Review on Centrifugation in the Pharmaceutical Industry. Am J Biomed Eng 2015;5:67-78.
- Pierce. Convert between times gravity (×g) and centrifuge rotor speed (RPM). Meridian 2005;0747:32869.
- Miron RJ, Pinto NR, Quirynen M, et al. Standardization of relative centrifugal forces in studies related to platelet-rich fibrin. J Periodontol 2019;90:817-20. [Crossref] [PubMed]
- Ghanaati S, Mourão CF. The Role of Centrifugation Process in the Preparation of Therapeutic Blood Concentrates: Standardization of the Protocols to Improve Reproducibility. Int J Growth Factors Stem Cells Dent 2019;1:32-7.
- Wend S, Kubesch A, Orlowska A, et al. Reduction of the relative centrifugal force influences cell number and growth factor release within injectable PRF-based matrices. J Mater Sci Mater Med 2017;28:188. [Crossref] [PubMed]
- Tunalı M, Özdemir H, Küçükodacı Z, et al. In vivo evaluation of titanium-prepared platelet-rich fibrin (T-PRF): a new platelet concentrate. Br J Oral Maxillofac Surg 2013;51:438-43. [Crossref] [PubMed]
Cite this article as: Herrera-Vizcaino C. Systematic review of platelet-rich fibrin (PRF) centrifugation protocols in oral and maxillofacial surgery and the introduction of AR2T3: an easy to remember acronym to correctly report vertical and horizontal PRF centrifugation. Front Oral Maxillofac Med 2023;5:5.