
Postoperative complications in patients undergoing major head and neck surgery requiring free tissue transfer—how do we improve?
Introduction
Postoperative complications in patients undergoing major head and neck surgery requiring free tissue transfer (HNSFTT) are prevalent, costly, and an adverse patient experience of surgical care (1). The aim of this report is to chronicle a decade in which postoperative complications have been studied, and care practices modified, with the aim of reducing both the severity and frequency of such events. We present the following article in accordance with the STROBE reporting checklist (available at https://fomm.amegroups.com/article/view/10.21037/fomm-20-58/rc).
Methods
Commencing in August 2009, prospective data on postoperative complications in a consecutive cohort of patients undergoing HNSFTT has been prospectively gathered in the oncological section of the West of Scotland Regional Maxillofacial Surgery service. To ensure as complete a data capture as possible on postoperative adverse events the health records of all patients was independently scrutinised. During this process putative predictive factors were also extracted. To ensure all eligible patients were included the database was cross referenced with the operating department record. Data on transfusion was cross referenced with the blood bank database. Data was collected up to July 2020. The outcomes of interest were major complications [Clavien-Dindo III and above (2)], wound complications, pulmonary complications, unscheduled return to the operating theatre in the post-operative period, and flap failure.
This study period was associated with both national and local quality improvement (QI)/patient safety initiatives. In 2008 the Scottish Patient Safety Programme (SPSP) initiative (3) was launched and had perioperative management, including implementation of the WHO surgical checklist, as one of its four key initial workstreams (4). The SPSP was designed to change the healthcare culture in NHS Scotland to one that has patient safety at its forefront. The introduction of the WHO surgical checklist featured, as did care bundles for peripheral and central venous catheters as well as urinary catheters. An early warning system to identify and protocolise management of the deteriorating patient was also implemented nationally. Each of these interventions used a multidisciplinary team approach to coordinate its introduction nationally with regional and local support. The implementation period was 2008–2010. Demonstrable improvements have occurred at a national level. For example, the implementation of the WHO Surgical Checklist was associated with a reduction in perioperative mortality across NHS Scotland that could not be accounted for by long-term trends (4). Simultaneously, Health Care Improvement Scotland provided mentorship to teams engaged in local QI.
A local team workstream initiative was commenced in late 2012 to establish an Enhanced Recovery After Surgery (ERAS) pathway for patients undergoing major head and surgery involving free tissue transfer. The full programme was implemented in March 2014. Over time minor modifications have been made. The current ERAS pathway is summarised (Figure 1). In 2017 a bundle of interventions was implemented specifically targeted at reducing postoperative lower respiratory tract infections. These comprised intraoperative lung protective ventilation volumes, the use of either PEEP or high flow oxygen as well as physiotherapy in the first 24 hours to ‘recruit’ atelectatic alveoli, more latterly use of tracheostomy tubes with a subglottic port for use prior to cuff deflation at 48 hours, early patient mobilisation and breathing exercises as well as extensive use of wound infusion catheters at flap donor sites (other than forearm) to reduce opiate requirements (4,5). The protocolisation of care in an ERAS pathway was conceived of as a vehicle to prevent ‘errors of omission’. To identify and decrease ‘errors of commission’ a re-structured mortality and morbidity meeting schedule was introduced in 2015. Meetings were held more regularly and followed a structured format with all cases experiencing a major complication (Clavien-Dindo III+) (2) discussed in detail. Simultaneous with this a UK national programme with leadership from the Royal Colleges of Surgeons was initiated to raise awareness of, and provide specific teaching on, the importance of non-technical skills in surgery (6) and human factors in clinical performance. Surgeons and anaesthetists (7) at all levels received instruction in this domain over the latter part of the study period.
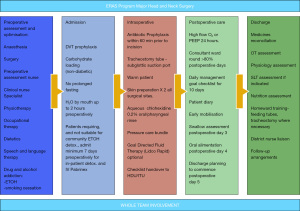
To gauge progress the study period was divided into three, each of which comprised 3 full calendar years. A 3-year time interval was selected because, for the volume of patients being treated, this was perceived to be a realistic time frame in which it was feasible to conduct repeated Donabedian plan-do-study-act QI cycles (8).
To investigate the possibility of bias the patients were compared across the three time periods in relation to markers of altered acute and chronic co-morbidity (age, gender, Adult Co-morbidity Evaluation-27 index, number of organ systems with co-morbidity present, Malnutrition Universal Screening Tool score, body mass index (BMI), WHO/ECOG performance status score, ASA score, smoking and alcohol status, presence or otherwise of diabetes, presence of occlusive vascular disease, preoperative white cell count, haemoglobin level (g/L), platelet count, serum albumin and C-reactive protein. Previous head and neck major surgery and/or radiotherapy was also compared. Surrogate markers of magnitude of surgical insult were also compared-tumour stage, site of primary disease, duration of procedure, use of tracheostomy, flap donor site and composition as well as limb versus truncal site, and requirement for blood transfusion.
Categorical putative predictive factors and outcome variables were compared across time periods using the Chi-Square test. Continuous variables compared with the t-test (normal distribution) and Mann-Whitney U test (non-parametric) as appropriate.
Statistical analysis
Categorical putative predictive factors and outcome variables were compared across time periods using the Chi-Square test. Continuous variables compared with the t-test (normal distribution) and Mann-Whitney U test (non-parametric) as appropriate. Putative categorical predictive variables were compared with the chi-square test. Statistical analysis was performed using SPSS-27 (IBM Corp., Armonk, NY, USA).
Ethical consideration
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the NHS Greater Glasgow and Clyde Clinical Governance Department as falling into the category of service evaluation clinical audit. Patient consent for the use of data for audit and research purposes was obtained. The patient dataset is registered on the institutional Information Asset Register and all data handling is in compliance with EU General Data Protection Regulations.
Results
The study population comprised 1,112 patients with 322 undergoing surgery in time period 1 (27/08/2009 to 16/12/2013), 360 in time period 2 (06/01/14 to 29/12/16) and 430 in time period 3 (05/01/17–30/06/20). There was no change over the three time periods in the gender profile, mean age of patients, tumour staging, Adult Co-Morbidity Evaluation-27 Score, ECOG/WHO Score, Malnutrition Universal Screening Tool preoperative score, ASA score, proportion of patients with diabetes, history of occlusive vascular disease ( coronary artery, cerebrovascular, or peripheral), smoking status, alcohol intake, pre-existing pulmonary disease, number of systems with co-morbidity, mean BMI, pre-operative white blood cell count, pre-operative haemoglobin, pre-operative platelet count, serum pre-operative C-reactive protein, pre-operative serum albumin, and duration of hospital stay (numbers of patients included and missing data shown on Table S1).
A significantly higher proportion of patients underwent surgery for a pharyngo-laryngeal defect after time period 1 and more had undergone prior head and neck radiotherapy and surgery in the later time periods (Table 1). There were changes in donor sites over the study period with decreasing use of rectus abdominis myocutaneous flaps and vascularised iliac crest flaps over successive time periods. In part these differences are explained by changes in surgical personnel, but also concern regarding abdominal wall integrity issues associated. The rectus abdominis flap has been supplanted by the use of the anterolateral thigh or latissimus dorsi myocutaneous flaps. In the latter two time periods the fibula and scapula osseous flaps have found favour over the iliac crest. Reflecting an individual surgeon’s preference, time period 2 saw a reduced use of the forearm donor site in favour of the lateral arm, largely reversed latterly. There has been a gradual reduction in the use of tracheostomy and perioperative blood transfusion (Table 1).
Table 1
| Variable | Time period, No. [%] | Statistic | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | |||
| Site | |||||
| Oral/oropharynx | 269 [83] | 267 [74] | 320 [74] | χ2=11.02, P=0.004 | |
| Larynx/hypopharynx | 14 [4.5] | 37 [10.5] | 28 [6.5] | χ2=9.43, P=0.009 | |
| Maxilla/skull base | 28 [9] | 34 [9.5] | 54 [12.5] | χ2=3.81, P=0.15 | |
| Non-UADT | 11 [3.5] | 22 [6] | 28 [7] | χ2=3.81, P=0.15 | |
| Totals | 322 | 360 | 430 | 1,112 | |
| Previous head and neck radiotherapy | |||||
| Yes | 30 [9] | 89 [25] | 64 [15] | χ2=31.05, df =3, P<0.001 | |
| No | 292 | 271 | 366 | ||
| Previous head and neck major surgery | |||||
| Yes | 39 [12] | 97 [27] | 128 [30] | χ2=35.12, df =3, P<0.001 | |
| No | 283 | 264 | 302 | ||
| Flap type | |||||
| Radial forearm | 178 [52] | 133 [33] | 213 [49] | χ2=32.40, P<0.001 | |
| Anterolateral thigh | 31 [9] | 55 [18] | 65 [15] | χ2=6.52, P=0.038 | |
| Rectus abdominis | 38 [11] | 12 [3] | 5 [1.2] | χ2=46.21, P<0.001 | |
| Latissimus dorsi | 8 [2] | 19 [5] | 28 [6.5] | χ2=7.34, P=0.026 | |
| Fibula | 23 [7] | 47 [12] | 45 [11] | χ2=5.57, P=0.061 | |
| Vascularised iliac crest | 44 [13] | 28 [7] | 8 [1.3] | χ2=36.50, P<0.001 | |
| Subscapular system composite | 19 [5.6] | 56 [14] | 56 [13] | χ2=15.51. P<0.001 | |
| Lateral arm | 0 | 24 [6] | 10 [2] | χ2=21.86, P<0.001 | |
| Miscellaneous | 1 | 5 | 4 | ||
| 2 flaps | 10 | 16 | 19 | χ2=1.17, P=0.55 | |
| Osseous flap | 80 [25] | 130 [36] | 122 [28] | χ2=11.66, df =3, P=0.009 | |
| Truncal vs. limb donor site | 102 [32] | 110 [30] | 97 [23] | χ2=9.86, df =3, P=0.020 | |
| Tracheostomy | 307 [95] | 306 [85] | 336 [78] | χ2=44.52, df =3, P<0.001 | |
| Transfusion | 152 [47] | 138 [38] | 113 [26] | χ2=41.00, df =3, P<0.001 | |
| Duration of anaesthesia [mean (Std. Dev.) minutes] | 613 (SD 136) | 699 (SD 176) | t=−6.8, P<0.001 | ||
| 699 (SD 176) | 640 (SD 159) | t=4.70, P=0.054 | |||
| 613 (SD 136) | 640 (SD 159) | t=2.42, P=0.016 | |||
UADT, upper aerodigestive tract.
There has been a reduction in wound complications in the latter time period (Table 2). Despite sustained and coordinated efforts there has been no reduction in major complications, unscheduled return to the operating room, and pulmonary complication. There has been a non-significant trend towards increased flap failures in tine period 3. This relates largely to a specific time period in 2018 with the direct antecedents being a number of high risk secondary reconstructive procedures in patients who had undergone previous major head and neck surgery with or without radiotherapy, coincident with the use of alternative donor sites perceived to have some advantages over more routinely deployed flaps. The ERAS team rapidly identified the problem and surgical decision making was reset and this has been associated with a subsequent reduction in flap failures to baseline levels.
Table 2
| Variable | Time period, No. [%] | Statistic | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Unscheduled return to operating room | 70 [22] | 95 [26] | 129 [30] | χ2=6.82, df =3, P=0.078 |
| Major complication (Clavien-Dindo Grade III, IV, V) | 117 [36] | 139 [38] | 158 [37] | χ2=1.04, df =3, P=0.792 |
| Wound complication | 153 [47.5] | 199 [55] | 149 [34] | χ2=31.07, df =3, P<0.001 |
| Pulmonary complication | 90 [28] | 97 [27] | 102 [24] | χ2=1.96, df =3, P=0.374 |
| Flap failures | 9 [3] | 14 [4] | 27 [6] | χ2=5.70, df =3, P=0.127 |
| Mortality (30 days or in-hospital) | 2 [0.6] | 4 [1.1] | 4 [0.9] | |
Discussion
Postoperative complications represent a poor patient experience, dramatically increased health care costs (1), and may impact upon overall survival in this population of patients (9). The outcome metrics selected are those which impact most on patients and service delivery while also, at least hypothetically, being amenable to QI initiatives. The prevalence of adverse events is high, both in the patient group reported here, and those reported by others (10-15). The literature also suggests variation in the prevalence of the adverse outcome metrics across institutions. This variation suggests considerable room for improvement in our own practice. Why then has our own experience over a decade, with a multidisciplinary local institutional, as well as healthcare system, coordinated effort been so unsuccessful at reducing the burden of patient suffering associated with this surgical endeavour?
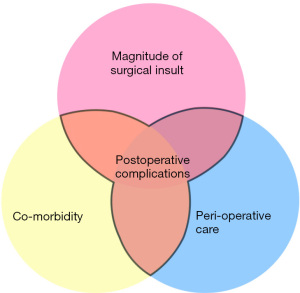
The current conceptual model to understand the genesis of postoperative complications describes an interaction between three groupings of variables. The altered acute and chronic physiology (co-morbidity) the patient brings to the operating table, the magnitude of the surgical insult imparted, and the entirety of the perioperative care package which will militate for or against those adverse events (Figure 2). Our lack of success certainly suggests that the former two groupings of variables have a dominant effect and the degree to which postoperative complications can be reduced by modification of the perioperative care limited. Simply stated—it is what we decide to do, who we select to do it to, and when we embark upon it that are likely to be at least as deterministic as how we go about it. To paraphrase the Agency for Healthcare Research and Quality definition: ‘the right care in the right patient at the right time in the right way’. This recognition is important. It shifts emphasis to the preoperative optimisation of patients, multidisciplinary evaluation to arrive at some estimate of what insult an individual will tolerate with a reasonable expectation of uncomplicated healing, and developing a surgical plan accordingly. Currently this remains largely dependent upon clinical intuition and experience but early attempts are being made to quantify patient physiology (14,16).
Our experience also indicates that an institution acting alone, even with sustained effort, may find it difficult to achieve rapid improvement. Inter-institutional comparative data, which is risk adjusted, allowing departments to compare outcomes for similar patient groups offers an opportunity for rapid dissemination of best practice and should be adopted by healthcare systems (17,18). Furthermore, the large volumes of data generated, with sufficient granularity, offer the best prospect of converting clinical intuition described above into clinical science with an empirical basis so procedures can be matched to patient tolerance.
UK healthcare surgical safety initiatives have thus far largely focused on reducing perioperative mortality and avoiding ‘never’ events. Where mortality rates are low shifting the focus to adverse events which are prevalent, measurable, as well as clinically important requires further emphasis in our view.
Considerable change occurred, both in the healthcare system and the individual surgical department, over the decade this study was conducted over. Evidence of bias exists with a significantly greater proportion of patients undergoing surgery in a relatively hostile surgical field (previous surgery and/or radiotherapy) in the latter part of the study. Furthermore, volume increased along with changes in surgical personnel. All these factors make it impossible to draw any firm conclusions about the benefit or otherwise of the interventions implemented in an attempt to reduce postoperative adverse events. Nevertheless, that is al ‘real world’ experience and, in the view of the authors further emphasises the need for collaborative QI work across healthcare systems.
Conclusions
Postoperative complications in patients undergoing head and neck surgery are somewhat predictable, they are prevalent, and recalcitrant. Healthcare systems need to adopt collaborative QI methodology to identify opportunities to reduce the prevalence and severity of postoperative adverse events. Some of this endeavour should focus on better defining the level of surgical insult that individual patients will tolerate and optimal procedures for specific clinical scenarios.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Michael Ho) for the series “Head and Neck Reconstruction” published in Frontiers of Oral and Maxillofacial Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://fomm.amegroups.com/article/view/10.21037/fomm-20-58/rc
Data Sharing Statement: Available at https://fomm.amegroups.com/article/view/10.21037/fomm-20-58/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://fomm.amegroups.com/article/view/10.21037/fomm-20-58/coif). The series “Head and Neck Reconstruction” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the NHS Greater Glasgow and Clyde Clinical Governance Department. This study received institutional review board approval as clinical audit and was not therefore regarded as an applicable study for research ethics committee scrutiny. Patient consent for the use of data for audit and research purposes was obtained. The dataset is registered on the institutional data asset register. All data handling is compliance with EU GDPR regulations and the UK Data Protection Act 2018.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- McMahon J, Handley TPB, Bobinskas A, et al. Postoperative complications after head and neck operations that require free tissue transfer - prevalent, morbid, and costly. Br J Oral Maxillofac Surg 2017;55:809-14. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Available online: https://ihub.scot/improvement-programmes/scottish-patient-safety-programme-spsp
- Ramsay G, Haynes AB, Lipsitz SR, et al. Reducing surgical mortality in Scotland by use of the WHO Surgical Safety Checklist. Br J Surg 2019;106:1005-11. [Crossref] [PubMed]
- Cassidy MR, Rosenkranz P, McCabe K, et al. I COUGH: reducing postoperative pulmonary complications with a multidisciplinary patient care program. JAMA Surg 2013;148:740-5. [Crossref] [PubMed]
- Agha RA, Fowler AJ, Sevdalis N. The role of non-technical skills in surgery. Ann Med Surg (Lond) 2015;4:422-7. [Crossref] [PubMed]
- Available online: https://www.rcoa.ac.uk/events/aae-anaesthetists-non-technical-skills-ants-0
- Busse R, Klazinga N, Panteli D, et al. Improving healthcare quality in Europe- characteristics, effectiveness and implementation of different strategies. Ch 2 Understanding healthcare quality strategies: a five-lens framework. World Health Organisation, Observatory on Health Systems and Policies, and OECD 2019; ISBN 978 92 890 5175 0, OECD ISBN 978 92 648 0590 3.
- Ch'ng S, Choi V, Elliott M, et al. Relationship between postoperative complications and survival after free flap reconstruction for oral cavity squamous cell carcinoma. Head Neck 2014;36:55-9. [Crossref] [PubMed]
- Cannady SB, Hatten KM, Bur AM, et al. Use of free tissue transfer in head and neck cancer surgery and risk of overall and serious complication(s): An American College of Surgeons-National Surgical Quality Improvement Project analysis of free tissue transfer to the head and neck. Head Neck 2017;39:702-7. [Crossref] [PubMed]
- Kiong KL, Lin FY, Yao CMKL, et al. Impact of neoadjuvant chemotherapy on perioperative morbidity after major surgery for head and neck cancer. Cancer 2020;126:4304-14. [Crossref] [PubMed]
- Eskander A, Kang SY, Tweel B, et al. Quality Indicators: Measurement and Predictors in Head and Neck Cancer Free Flap Patients. Otolaryngol Head Neck Surg 2018;158:265-72. [Crossref] [PubMed]
- Marttila E, Thorén H, Törnwall J, et al. Complications and loss of free flaps after reconstructions for oral cancer. Br J Oral Maxillofac Surg 2018;56:835-40. [Crossref] [PubMed]
- Chen Y, Cao W, Gao X, et al. Predicting postoperative complications of head and neck squamous cell carcinoma in elderly patients using random forest algorithm model. BMC Med Inform Decis Mak 2015;15:44. [Crossref] [PubMed]
- Wu CC, Lin PY, Chew KY, et al. Free tissue transfers in head and neck reconstruction: complications, outcomes and strategies for management of flap failure: analysis of 2019 flaps in single institute. Microsurgery 2014;34:339-44. [Crossref] [PubMed]
- Lalabekyan BB, Tetlow N, Moonesinghe R, et al. Cardiopulmonary exercise testing and cardiopulmonary morbidity in patients undergoing major head and neck surgery. Br J Oral Maxillofac Surg 2021;59:297-302. [Crossref] [PubMed]
- Cohen ME, Liu Y, Ko CY, et al. Improved Surgical Outcomes for ACS NSQIP Hospitals Over Time: Evaluation of Hospital Cohorts With up to 8 Years of Participation. Ann Surg 2016;263:267-73. [Crossref] [PubMed]
- Nikolian VC, Regenbogen SE. Statewide Clinic Registries: The Michigan Surgical Quality Collaborative. Clin Colon Rectal Surg 2019;32:16-24. [Crossref] [PubMed]
Cite this article as: McMahon J, Abraham J, McMahon GC, Zubair F. Postoperative complications in patients undergoing major head and neck surgery requiring free tissue transfer—how do we improve? Front Oral Maxillofac Med 2022;4:12.

