
Antimicrobial surface treatment of titanium dental implants: a narrative review between 2011 and 2021
Introduction
The rational for this review is to determine progress on the critical need of curtailing or preventing peri-implant disease from oral biofilm on exposed titanium surface of dental implants. The incidence of peri-implant disease increases over time with long term studies showing an incidence exceeding 20% (1). A 9-year study in 588 patients showed a 45% incidence of bleeding on probing associated with bone loss including a 14.5% incidence of greater than 2 mm bone loss attributed to peri-implant disease (2). The net effect of periimplantitis is exposed titanium surface to the oral cavity from peri-implant bone loss which tends to progress long-term to implant failure (3-7).
Though there are many confounding risk factors, the dental profession is left with the problem of how to manage exposed titanium surfaces which accumulate difficult to remove microbial biofilm (8).
Hickok et al. described the potential impact of antimicrobial action at the implant surface delineating the use of three approaches including enhanced nano-topography, elution of antimicrobials to retard bacterial adhesion and bonding of pharmaceutical agents to the surface all of which at present have failed to affect a long-term antimicrobial function (9).
The key narrative review question is: Has there been an effective titanium surface treatment with long term antimicrobial activity to prevent peri-implant disease? And, if not: Is there a promising technology published that appears to be able to accomplish a significant reduction in peri-implant disease?
Whatever the exact mechanism of peri-implant de-osseointegration, which might include foreign body debris such as retained cement or sheared titanium particulate matter, once bone attachment is lost the device withstands continuous bacterial insult leading to osteoclastic bone resorption and progressive bone loss which likely does not reform even if the titanium surface is decontaminated and bone grafted (2-18).
Decontamination and regrafting of lost bone around titanium implants is possible but with highly variable result, not verified longitudinally and requires relatively invasive surgery (17,18). So, the loss of bone around implants even if treatable and manageable becomes a risk for implant failure (3-7).
There are five clinical findings associated with exposed implant surface which impact the dental health of a patient as follows:
- Continuous requirement for additional hygiene measures;
- Gingival margin instability;
- Circumferential bone loss progression;
- Papillary height instability;
- Implant source extension of peri-implant disease.
The purpose of this article is to highlight the problem of exposed titanium in the mouth and the possible role of antimicrobial treatments on the existence and progression of peri-implant disease. We present the following article in accordance with the Narrative Review reporting checklist (available at https://fomm.amegroups.com/article/view/10.21037/fomm-21-62/rc).
Methods
Though the manuscript is not a systematic review but a narrative review, we searched relevant literature with the following consideration (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | 12/1/2021 |
| Databases and other sources searched | PubMed |
| Search terms used | Anti-bacterial treatment of titanium dental implants |
| Timeframe | 2011–2021 |
| Inclusion and exclusion criteria | Antimicrobial coating of titanium dental implants |
| Selection process | ADA librarian selected articles based on MeSH search criteria |
ADA, American Dental Association.
Results
There were 16 articles that met the initial criteria including 3 review articles for various antimicrobial coating treatments of titanium dental implants listed in Figure 1. Though these articles showed evidence of some antimicrobial efficacy either in vivo or in vitro there was no clear evidence for prevention of biofilm formation and therefore prevention of peri-implantitis. The number of articles, therefore, that showed a sustained antimicrobial activity for clinically significant efficacy was zero.
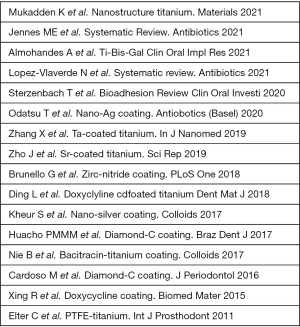
Key findings of literature review
The key findings were 16 articles including 3 review article that met the criteria of which none showed clinical efficacy to prevent peri-implant disease.
Limitations of research reviewed
The limitation of the research review was a search for clinically sustained efficacy of any significance which there was none. The vast majority of the articles that reported antimicrobial effect were not in vivo suggesting a much too abbreviated search criteria if in vitro study was excluded.
Without antimicrobial-titanium function, what then?
The lack of antimicrobial function for a titanium implant surface leaves the clinician with the critical management challenge of reduced expectation for health, function and esthetics of the dental implant restoration as follows.
Five clinical findings associated with exposed implant surface which impact dental implant health
Continuous requirement for additional hygiene measures
Once an exposed implant surface occurs, particularly if gingival recession occurs, the rough surface becomes a strong plaque retainer and is difficult to keep clean. Inadequate oral hygiene coupled with sucrose intake leads to bacterial synthesis of insoluble exopolysaccharides (EPS) polymers which strengthen the biofilm making it resistant to antibiotics and leading to a dysbiosis anaerobic environment. In one study an EPS enriched environment favored growth of strict anaerobic species such as Porphyromonas gingivalis as biofilm transitioned from a commensal aerobic to a pathogenic anerobic milieu (19).
Smoothing off the surface of the exposed implant, removing screw threads and rough surface leaving a polished surface is somewhat less likely to accumulate plaque as the adhesion of bacteria is promoted by rough surfaces (18,20-22).
The process of macro-modification of exposed titanium, however, will likely add peri-implant titanium debris which could aggravate peri-implantitis similar to the use of ultrasonic scalers which have been shown to add titanium matter from titanium surfaces potentially leading to osteoclasis and foreign body reaction as is found with wear particles found in orthopedic metallosis. More important is to prevent pocket formation or reduce gingival pockets to 3 mm in order to minimize inflammation and pocket depth progression by promoting an aerobic environment (23-25).
A facial bone graft done at the time of implant placement might be lost from peri-implant inflammation leaving a dehiscence defect that can been debrided and regrafted with a hard or soft tissue graft in an effort to reduce inflammation (16,17). Bone grafting done successfully can arrest peri-implant disease extension but is not yet highly predictable (15).
Once exposed titanium occurs adjacent to another implant or close to a tooth risk for extension of the inflammatory lesion to adjacent attachment is possible requiring careful daily hygiene measures and other preventive care (26).
Gingival margin instability
Once marginal bone is lost and the titanium surface is not covered with bone gingival recession may occur. Recession is somewhat dependent on timing of implant placement, horizontal implant position and gingival biotype, with thin biotype individuals more susceptible to recession. Though there is not a one to one correlation between implant exposure and gingival recession there is a correlation, and sometimes recession is the initial finding when peri-implant disease is present (26-30).
Circumferential bone loss progression
Periodontal disease on teeth is site specific while peri-implantitis is implant specific. This is because there is a different pathway for progression of inflammation. That is, on teeth, Sharpey’s fibers insert perpendicularly into the cementum and once interrupted the pathway of the inflammation can go down the side of the tooth vertically via the ligament space but it can also go sideways and form a circumferential defect. With implants lesions are circumferential and not vertical for the most part as once the inflammatory infiltrate gets past the junctional epithelium the easiest pathway is horizontal, starting around the top of the implant, because the connective tissue fibers adhere to the implant with parallel fibers without insertion into titanium. Furthermore, peri-implant lesion accelerates in a non-linear fashion with greater inflammatory findings than found with typical periodontitis lesions including marked vascular proliferation, lesions extending to a position that is apical of the pocket epithelium and lesions not being surrounded by non-infiltrated connective tissue as is found in periodontitis (14,31,32).
Methods to eliminate pockets around implants must address this and can involve osseous recontouring, apically repositioned flaps or decontamination of the implant surface and bone grafting, the latter usually requires a barrier membrane and possible submersion of the implant for some time (33,34).
Papillary height instability
The gingival papilla has complex attachment between teeth that include vertical support from supra-crestal gingival fibers. There fibers are absent around implants and are especially deficient between two implants. However, another factor of importance is the osseous foundation of a papillae. When a muco-periosteal flap, including the papilla is reflected and re-sutured into place, if the bone support for a papilla is present the papillae will recover its initial anatomical presence. However, if the bone is absent significantly papillary height will not recover. So, the osseous foundation of the papilla is highly important when it comes to implants placed in close proximity to teeth or to each other. Current thinking is that an implant should be placed 2 mm away from a tooth and 3 mm away from each other. As crestal bone loss occurs around an implant, initially the proximate papilla may remain, but as more and more bone is undermined, papillary support is lost (35-38).
One of the most difficult things to treat is absence of adjacent maxillary lateral and central incisors. This is very difficult to treat with side-by-side implants because the papilla between two implants is difficult to mimic when compared to the opposite dentate setting. Therefore, practitioners have settled for placing one implant and cantilevering the lateral incisor to address this. Once two side by side implants have become involved with peri implant bone loss in this setting the papilla between them will be lost and will require much effort to recover, if possible at all (39-42).
Implant source extension of peri implant disease
Osseous infection that is chronic such as peri-implantitis may spread to adjacent healthy structures such as an adjacent implant or tooth. Therefore, implant source contamination from the abutment connection and implant surface when in proximity to another implant is an important consideration. For example, complete arch implant placement where multiple implants are placed in close proximity, although biomechanically sound, may add long term risk if implant connections are not accurate or implants are placed too close to one another (41,43).
How close should one implant be to another? Depending on the implant system three mm has been suggested. There is no scientific formula but if the zone of inflammation is about 1.5 mm and two adjacent implants are less than 3 mm apart the zones of inflammation may combine to create a larger lesion. This then, suggests a minimum (37,38,44-46).
The implant-abutment junction
The one-piece or tissue level implant has the least peri-implant inflammation when compared to standard bone level, two-piece implants. Moreover, abutment connections vary with efficacy at maintaining a sterile seal between the implant and abutment. Figure 2 shows a very tight, 5 micron seal. Figure 3 shows an example of an early development abutment connection gap approaching 20 microns that allowed for bacterial percolation during functional movement. Whereas the Figure 2 implant-abutment junction shows the picture of an implant abutment interface gap which is smaller than the size of E. faecalis which is 1/2 micron in size. Hardware precision for bone level implants, therefore, is an important consideration for bacterial source for peri-implant disease (47,48).
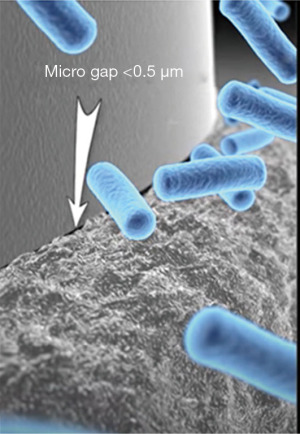
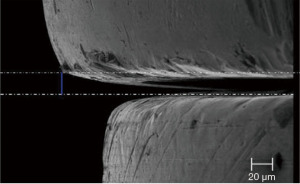
When gap sizes exceed 2 to 3 microns, all oral bacterial species in the oral cavity are able to ingress/egress the implant abutment junction to populate the “zone of inflammation” potentially leading to dysbiosis.
In addition to a precision abutment connection, the use of platform switch to keep the zone of inflammation away, the use of platform bone switch to increase bone mass at the cervical wall around an implant, a self-cleanable restoration and good oral hygiene measures—all impact the minimum distance required between implants. Great care should be taken in the decision to place side by side implants or placement of an implant into a tight interdental space such as a single lower incisor site that might encroach on the periodontal ligament space as the implant in itself and abutment interface in particular are a potential source risk for subgingival bacterial contamination (47-53).
Discussion and possible future solutions
The results of the key questions showed no clear evidence for any technique or process eliminating or curtailing long term contamination for titanium dental implants.
Given these risks of peri-implantitis in light of the findings discussed above, a change in the titanium surface capacity to become less likely to inflame or chronically infect local tissue could profoundly impact dentistry and ultimately change the way implants are placed including proximity to teeth and each other. In fact, there may be clinical situations in which implants are relatively contraindicated in which an implant that had antimicrobial capacity could then be implemented. There are four examples of this including: (I) patients with partial edentulism and recurrent periodontitis that is chronic but still not to the point of requiring removal of remaining teeth; (II) patients with osseous vascularity problems such as found in radiation treatment, bisphosphonate osteonecrosis history or high pack history tobaccos use; (III) multiple implant failure history and (IV) patients with failed bone grafting history particularly extensive vertical bone grafting that might be relatively unstable bone leading to remodeling exposure of implants over time—all these become relative indications for the use of an anti-microbial implant (54-57).
Periodontitis and dental implants
Perhaps the greatest indication for an antimicrobial implant is a history of periodontitis or ongoing periodontitis where implants are desired. The success rate of implants in partially edentulous patients who have had periodontitis can be nearly equal to patients without a periodontitis history if oral hygiene measures are strictly maintained. However, in patients with severe periodontitis, despite treatment and maintenance measures being optimal there is still a significantly increased incidence of peri implant disease over periodontally healthy patients.
So an antimicrobial implant will certainly open up opportunity for more frequent use in the compromised setting of previous or even present periodontal disease (58).
Compromised bone biology
Radiation treatment, bisphosphate history and tobacco use are patient histories that indicate possible compromise to bone biology (59-61).
Radiation therapy causes vascular compromise to bone such that reflecting a soft tissue flap may expose non-vital bone that does not revascularize adequately leading to dehiscence and osseous necrosis. These settings are obviously not indicated for osteointegration unless subthreshold radiation doses or possibly the use of hyperbaric oxygen therapy are done to improve bone vitality. An early event leading to peri-implant bone loss in the radiation case may actually be from fatigue failure of nonvital bone with subsequent bone loss leading to exposure of titanium and secondarily, periimplantitis, which could be curtailed by the use of an antimicrobial implant (59).
Parenteral or prolonged oral bisphosphonate treatment either prior to or subsequent to implant placement can lead to loss of osteointegration due to osseous necrosis, a cofactor being secondary infection from exposed titanium implants. Placement of implants in patients on drug holiday or with a significant history osteoporosis treatment may warrant the use of antimicrobial implants (60).
The use of tobacco is important as it interferes with osseous vascularity as well as soft tissue attachment. The incidence of bone loss in patients who use tobacco is greater as is the incidence of periimplantitis and implant failure. Therefore, the option of using an antimicrobial implant may have a distinct advantage in tobacco users (61).
Adjacent disease
The need for an antimicrobial implant is not strictly for iatrogenic or compromised biology reasons. Inadvertent bone loss can occur physiologically or due to adjacent tooth infection transmitted to an adjacent implant which might include periodontitis, gingival abscess, or endodontic abscess.
Well controlled studies are interesting and important but often do not take into account the human factor leading to poorly placed implants or even iatrogenic placement including placement in non-ideal settings where implants might not thrive as well. In a way, these controlled studies remove risk and poor methodology as a matter of course leading to idealized and perhaps uncommon results. Therefore, the use of an anti-microbial implant could function as a failsafe mechanism in prevention or extension of peri-implant disease process in any practitioner’s practice. The absolute indication for an anti-microbial implant then is for any oral penetrating dental implant which has a potential for inflammatory, traumatic or physiologic bone loss (62).
Coatings
The use of an antibacterial coating for a two-piece dental implant might optimally be at the abutment-implant connection, the hardware interface, instead of at the bone-implant interface along the sides of the implant where osseointegration occurs. Hypothetically, if this connection could be coated to prevent formation of biofilm this might go a long way towards prevention of peri-implant disease. Still, once implant surface of an implant becomes exposed for whatever reason coating technology would be needed there as well.
Antimicrobial coating of dental implants could occur in multiple ways including the use of antimicrobial peptides (AMPs), slow-release antibiotics, addition of heavy metals such as silver, and modification of titanium surface incorporating the use of antimicrobial organic compounds. However, almost any type of coating could potentially interfere with osseointegration (54,57).
Coating for implants is classified into passive or active depending on their mode of action. Passive coatings do not release product into the surrounding tissues whereas active coatings release agents into the peri implant environment. Examples of active coatings are antibiotics, metal ions and functional peptides that downregulate infection (63-67).
Passive coatings
Antimicrobial surfaces can be obtained by modifying the crystalline structure of the oxide layer. For example, bacterial adhesion is inhibited by ultraviolet light irradiation which hydrophilizes the titanium oxide surface. This process does not interfere with osseointegration (67,68).
Other passive coatings such as polymer coatings like polyethylene glycol inhibit bacteria when applied to titanium surface but osteoblast function is impaired requiring the use of additional bioactive molecules to restore cell function. Albumin has also been shown to inhibit bacterial adhesion on titanium surface (68).
Active coatings
The idea of coating implants with an antibiotic comes in part from the successful use of perioperative antibiotics. This includes the use of prophylaxis, intra-operative and post-operative prescription all of which have shown positive effect on the healing and survival of osseointegrated implants. Antibiotics have therefore been covalently linked to implant surfaces however, optimal release kinetics remains unresolved. And, once the titer of the antibiotic depot falls below a certain threshold concentration efficacy is lost. So antibiotic release remains a timing delivery quandary (69,70).
Jennes’ and Lopez-Valverde’s systemic reviews of various antimicrobial coating methods found only 9 and 6 articles (4 in the last ten years) respectively that met their criteria for inclusion, most of which were invitro studies, none showing efficacy for peri-implant disease (71-88).
Antimicrobial organics
AMPs are naturally occurring substances that target and kill a broad spectrum of gram-positive and gram-negative bacteria, fungi, and viruses by disrupting cell membranes and causing cell lysis. AMPs are not prone to the development of pathogen resistance like antibiotics. AMPs can be active when free in solution or adsorbed onto a surface such as titanium and have been shown to have antimicrobial efficacy against S. aureus and P. aeruginosa. Streptococcal collagen-mimetic protein coating has also been shown to reduce bacterial adherence of S. aureus and S. epidermidis. Although AMPs show a low tendency to induce resistances, more and more AMPs are losing their antimicrobial effectiveness against various bacterial strains over time reducing the incentive to commercially fabricate bioactive coatings using AMPs (89-93).
Bactericidal nanoparticles
Nanoparticles ranging from 1–100 nm incorporating copper, zinc, magnesium and especially silver and gold display antimicrobial activity and are therefore possible candidate molecules for antimicrobial implant surface modifications. Nanomaterials are used to create unique surfaces with altered physical and chemical characteristics but one major toxicological concern is that nanoparticles are easily phagocytized and may affect intracellular function. In procaryotes nanoparticles disrupt cell membranes but can also cause inhibition of DNA replication by binding to DNA. Interestingly, antimicrobial specificity varies with the various metal ions leading to differing bacteria biofilm constituents that are altered. Free particles of titanium at nanoscale also effect cellular response biology (54,57,64,75).
Summary
A narrative review of the literature for the last ten years showed minimal or absence of antimicrobial action with time as most all studies were in vitro and not in vivo. There were no sustained treatments that were shown to prevent peri-implant inflammation and in fact, transient effects such as the use of antibiotic coatings, though pharmacodynamic had end-point inefficacy.
The need for antimicrobial capacity for oral titanium implants is well know because of the likelihood that over time titanium implant surfaces become exposed in the oral cavity. The time scale of these events can take years, something not addressed in any of these studies.
Once titanium becomes exposed in the mouth increased efforts are required for hygiene maintenance to mitigate increased risk for peri-implantitis and late term implant failure.
Though any titanium device in the body is at risk for bacterial contamination from hematogenous etiology with oral penetrating devices there is particular risk for local contamination.
Antimicrobial strategy, therefore, remains an important area of investigation and is an ongoing need to curtail the nearly endemic prevalence of peri-implant disease in this important discipline of dentistry.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Frontiers of Oral and Maxillofacial Medicine for the series “Current Advances in Treatment of Peri-Implantitis”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://fomm.amegroups.com/article/view/10.21037/fomm-21-62/rc
Conflicts of Interests: All authors have completed the ICMJE uniform disclosure form (available at https://fomm.amegroups.com/article/view/10.21037/fomm-21-62/coif). The series “Current Advances in Treatment of Peri-Implantitis” was commissioned by the editorial office without any funding or sponsorship. OTJ served as the unpaid Guest Editor of the series and serves as the unpaid editorial board member of Frontiers of Oral and Maxillofacial Medicine from December 2021 to November 2023. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fu JH, Wang HL. Breaking wave of peri-implantitis. Periodontol 2000 2020;84:145-60. [Crossref] [PubMed]
- Esposito M, Worthington HV, Thomsen P, et al. Interventions for replacing missing teeth: different types of dental implants. Cochrane Database Syst Rev 2003;CD003815. [PubMed]
- Heitz-Mayfield LJ, Mombelli A. The therapy of peri-implantitis: a systematic review. Int J Oral Maxillofac Implants 2014;29:325-45. [Crossref] [PubMed]
- Heitz-Mayfield LJ, Needleman I, Salvi GE, et al. Consensus statements and clinical recommendations for prevention and management of biologic and technical implant complications. Int J Oral Maxillofac Implants 2014;29:346-50. [Crossref] [PubMed]
- Mombelli A, Müller N, Cionca N. The epidemiology of peri-implantitis. Clin Oral Implants Res 2012;23:67-76. [Crossref] [PubMed]
- Esposito M, Grusovin MG, Worthington HV. Interventions for replacing missing teeth: treatment of peri-implantitis. Cochrane Database Syst Rev 2013;CD003878. [PubMed]
- Filipović U, Dahmane RG, Ghannouchi S, et al. Bacterial adhesion on orthopedic implants. Adv Colloid Interface Sci 2020;283:102228. [Crossref] [PubMed]
- Bedi RS, Beving DE, Zanello LP, et al. Biocompatibility of corrosion-resistant zeolite coatings for titanium alloy biomedical implants. Acta Biomater 2009;5:3265-71. [Crossref] [PubMed]
- Hickok NJ, Shapiro IM, Chen AF. The Impact of Incorporating Antimicrobials into Implant Surfaces. J Dent Res 2018;97:14-22. [Crossref] [PubMed]
- Brandao ML, Vettore MV, Vidigal GM Jr. Peri-implant bone loss in cement and screw retained prostheses: systematic review and meta-analysis. J Clin Periodontol 2013;40:287-95. [Crossref] [PubMed]
- Fretwurst T, Nelson K, Tarnow DP, et al. Is Metal Particle Release Associated with Peri-implant Bone Destruction? An Emerging Concept. J Dent Res 2018;97:259-65. [Crossref] [PubMed]
- Romanos GE, Fischer GA, Delgado-Ruiz R. Titanium Wear of Dental Implants from Placement, under Loading and Maintenance Protocols. Int J Mol Sci 2021;22:1067. [Crossref] [PubMed]
- Lee CT, Huang YW, Zhu L, et al. Prevalences of peri-implantitis and peri-implant mucositis: systematic review and meta-analysis. J Dent 2017;62:1-12. [Crossref] [PubMed]
- Schwarz F, Derks J, Monje A, et al. Peri-implantitis. J Periodontol 2018;89:S267-90. [Crossref] [PubMed]
- Derks J, Schaller D, Håkansson J, et al. Effectiveness of Implant Therapy Analyzed in a Swedish Population: Prevalence of Peri-implantitis. J Dent Res 2016;95:43-9. [Crossref] [PubMed]
- Sanz-Sánchez I, Carrillo de Albornoz A, Figuero E, et al. Effects of lateral bone augmentation procedures on peri-implant health or disease: A systematic review and meta-analysis. Clin Oral Implants Res 2018;29:18-31. [Crossref] [PubMed]
- Larsson L, Decker AM, Nibali L, et al. Regenerative medicine for periodontal and peri implant diseases. J Dent Res 2016;95:255-66. [Crossref] [PubMed]
- Lasserre JF, Brecx MC, Toma S. Implantoplasty Versus Glycine Air Abrasion for the Surgical Treatment of Peri-implantitis: A Randomized Clinical Trial. Int J Oral Maxillofac Implants 2020;35:197-206. [Crossref] [PubMed]
- Costa RC, Souza JGS, Bertolini M, et al. Extracellular biofilm matrix leads to microbial dysbiosis and reduces biofilm susceptibility to antimicrobials on titanium biomaterial: An in vitro and in situ study. Clin Oral Implants Res 2020;31:1173-86. [Crossref] [PubMed]
- Katsikogianni M, Missirlis YF. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactions. Eur Cell Mater 2004;8:37-57. [Crossref] [PubMed]
- Kocacvic D, Pratnekar R, Godic-Torkar K, et al. Influence of polyelectrolyte multilayer properties of bacterial adhesion capacity. Polymers 2016;8:345. [Crossref] [PubMed]
- Bohinc K, Drazic G, Abram A, et al. Metal surface characteristics dictate bacterial adhesion capacity. Int J Adhes 2016;68:39-46. [Crossref]
- Eger M, Sterer N, Liron T, et al. Scaling of titanium implants entrains inflammation induced osteolysis. Sci Rep 2017;7:39612. [Crossref] [PubMed]
- Ingham E, Fisher J. The role of macrophages in osteolysis of total joint replacement. Biomaterials 2005;26:1271-86. [Crossref] [PubMed]
- St Pierre CA, Chan M, Iwakura Y, et al. Periprosthetic osteolysis: characterizing the innate immune response to titanium wear-particles. J Orthop Res 2010;28:1418-24. [Crossref] [PubMed]
- Figuero E, Graziani F, Sanz I, et al. Management of peri-implant mucositis and peri-implantitis. Periodontol 2000 2014;66:255-73. [Crossref] [PubMed]
- Mazzotti C, Stefanini M, Felice P, et al. Soft tissue dehiscence coverage at peri-implant sites. Periodontol 2000 2018;77:256-72. [Crossref] [PubMed]
- Frizzera F, Oliveira GJPL, Shibli JA, et al. Treatment of peri-implant soft tissue defects: a narrative review. Braz Oral Res 2019;33:e073. [Crossref] [PubMed]
- Garabetyan J, Malet J, Kerner S, et al. The relationship between dental papilla and dental implant mucosa around single implants in the esthetic area: A retrospective study. Clin Oral Implants Res 2019;30:1229-37. [Crossref] [PubMed]
- Buser D, Chappuis V, Belser UC, et al. Implant placement post extraction in esthetic single tooth sites: when immediate, when early, when late? Periodontol 2000 2017;73:84-102. [Crossref] [PubMed]
- Carcuac O, Berglundh T. Composition of human peri-implantitis and periodontitis lesions. J Dent Res 2014;93:1083-8. [Crossref] [PubMed]
- Derks J, Schaller D, Håkansson J, et al. Peri-implantitis - onset and pattern of progression. J Clin Periodontol 2016;43:383-8. [Crossref] [PubMed]
- Chan HL, Lin GH, Suarez F, et al. Surgical management of peri-implantitis: a systematic review and meta-analysis of treatment outcomes. J Periodontol 2014;85:1027-41. [Crossref] [PubMed]
- Khoury F, Keeve PL, Ramanauskaite A, et al. Surgical treatment of peri-implantitis - Consensus report of working group 4. Int Dent J 2019;69:18-22. [Crossref] [PubMed]
- Papapanou PN, Sanz M, Buduneli N, et al. Periodontitis: Consensus report of workshop 2 of the 210 World Workshop on the classification of periodontal and peri-implant diseases and conditions. Periodontol 2018;89 Suppl 1:S173-82.
- Cortellini P, Tonett MS, Lang NP, et al. The simplified papilla preservation flap in the regeneration treatment of deep intra bony defects: clinical outcomes and postoperative morbidity. J Periodontol 2001;72:1702-12. [Crossref] [PubMed]
- Tarnow DP, Mager AW, Fletcher PJ. The effect of the distance from the contact point to the crest of bone on the presence or absence of the inter-proximate dental papilla. J Periodontol 1992;63:995-6. [Crossref] [PubMed]
- Scarano A, Assenza B, Piatelli M, et al. Inter-implant distance and crestal bone resorption: a histological study in the canine mandible. Clin Implant Dent Relat Res 2004;6:150-6. [Crossref] [PubMed]
- Tarnow DP, Cho SC, Wallace SS. The effect of inter-implant distance on the height of inter-implant bone crest. J Periodontol 2000;71:546-9. [Crossref] [PubMed]
- Tarnow D, Elian N, Fletcher P, et al. Vertical distance from the crest of bone to the height of the interproximal papilla between adjacent implants. J Periodontol 2003;74:1785-8. [Crossref] [PubMed]
- Elian N, Bloom M, Dard M, et al. Radiological and micro-computed tomography analysis of the bone at dental implants inserted 2, 3 and 4 mm apart in a minipig model with platform switching incorporated. Clin Oral Implants Res 2014;25:e22-9. [Crossref] [PubMed]
- Needleman I, Chin S, Obrien T, et al. Systematic review of outcome measurements and reference groups(s) to evaluate and compare implant success and failure. J Clin Periodontol 2012;39:122-32. [Crossref] [PubMed]
- Chrcanovic BR, Kisch J, Albrektsson T, et al. Analysis of risk factors for cluster behavior of dental implant failures. Clin Implant Dent Relat Res 2017;19:632-42. [Crossref] [PubMed]
- Koutouzis T. Implant-abutment connection as a contributing factor to peri-implant diseases. Periodontol 2000 2019;81:152-66. [Crossref] [PubMed]
- Lauritano D, Moreo G, Lucches A, et al. The impact of implant abutment connection on clinical outcomes and microbial colonization: A narrative review. Materials (Basel) 2020;13:1131. [Crossref] [PubMed]
- Romanos GE, Delgado-Ruiz R, Sculean A. Concepts for prevention of complications in implant therapy. Periodontol 2000 2019;81:7-17. [Crossref] [PubMed]
- Strietzel FP, Neumann K, Hertwl M. Impact of platform switching on marginal peri-implant bone level changes. A systematic review and meta-analysis. Clin Oral Implants Res 2015;26:342-58. [Crossref] [PubMed]
- Caricasulo R, Malchiodi L, Ghensi P, et al. The influence of implant-abutment connection to peri-implant bone loss: A systematic review and meta-analysis. Clin Implant Dent Relat Res 2018;20:653-64. [Crossref] [PubMed]
- Zandim-Barcelos DL, Carvalho GG, Sapata VM, et al. Implant-based factor as possible risk for peri-implantitis. Braz Oral Res 2019;33:e067. [Crossref] [PubMed]
- Danza M, Quaranta A, Carinci F, et al. Biomechanical evaluation of dental implants in D1 and D4 bone by Finite Element Analysis. Minerva Stomatol 2010;59:305-13. [PubMed]
- Danza M, Riccardo G, Carinci F. Bone platform switching: a retrospective study on the slope of reverse conical neck. Quintessence Int 2010;41:35-40. [PubMed]
- Danza M, Ricardo G, Carnicini F. Bone platform switching in a retrospective study on the slope of reverse conical neck. Quintessence Int 2010;41:35-40. [PubMed]
- Danza M, Fromovich O, Guidi R, et al. The clinical outcomes of 234 spiral family implants. J Contemp Dent Pract 2009;10:E049-56. [Crossref] [PubMed]
- Goodman SB, Yao Z, Keeney M, et al. The future of biologic coatings for orthopedic implants. Biomaterials 2013;34:3174-83. [Crossref] [PubMed]
- Penn-Barwell JG, Bennett PM, Mortiboy DE, et al. Factors influencing infection in 10 years of battlefield open tibia fractures. Strategies Trauma Limb Reconstr 2016;11:13-8. [Crossref] [PubMed]
- Zimmerli W, Trampuz A. Implant-associated infection. Biofilm infections. Springer Clin Orthop Relat Res 2016;474:2394-404. review.
- Romano CL, Scarponi S, Gallazzi E, et al. Antibacterial coating of implants in orthopedics and trauma: a classification proposal in an evolving panorama. J Orthop Surg Res 2015;10:157. [Crossref] [PubMed]
- Laskin DM, Dent CD, Morris HF, et al. The influence of preoperative antibiotics on success of endosseous implants at 36 months. Ann Periodontol 2000;5:166-74. [Crossref] [PubMed]
- Harrison JS, Stratemann S, Redding SW. Dental implants for patients who have had radiation treatment for head and neck cancer. Spec Care Dentist 2003;23:223-9. [Crossref] [PubMed]
- Vohra F, Al-Rifaiy MQ, Almas K, et al. Efficacy of systemic bisphosphonate delivery on osseointegration of implants under osteoporotic conditions: lessons from animal studies. Arch Oral Biol 2014;59:912-20. [Crossref] [PubMed]
- Javed F, Rahman I, Romanos GE. Tobacco-product usage as a risk factor for dental implants. Periodontol 2000 2019;81:48-56. [Crossref] [PubMed]
- Kalaivin G, Blaaji VR, Manikandan D, et al. Expectation and reality of guided implant surgery protocol using computer-assisted static and dynamic navigation system at present scenario: Evidence-based literature review. J Indian Soc Periodontol 2020;24:398-408. [Crossref] [PubMed]
- Zhang BGX, Meyers DE, Wallace GG, et al. Bioactive coatings for orthopedic implants-recent trends in development of implant coatings. Int J Mol Sci 2014;15:11878-921. [Crossref] [PubMed]
- Liu Y, Zheng Z, Zara JN, et al. The antimicrobial and osteoinductive properties of silver nanoparticle/poly DL lactate co-glycolic acid-coated stainless steel. Biomaterials 2012;33:8745-56. [Crossref] [PubMed]
- Zhang L, Pei J, Wang H, et al. Facile Preparation of Poly(lactic acid)/Brushite Bilayer Coating on Biodegradable Magnesium Alloys with Multiple Functionalities for Orthopedic Application. ACS Appl Mater Interfaces 2017;9:9437-48. [Crossref] [PubMed]
- Zhao L, Chu PK, Zhang Y, et al. Antibacterial coatings on titanium implants. J Biomed Mater Res B Appl Biomater 2009;91:470-80. [Crossref] [PubMed]
- Raphel J, Holodniy M, Goodman SB, et al. Multifunctional coatings to simultaneously promote osseointegration and prevent infection of orthopedic implants. Biomaterials 2016;84:301-14. [Crossref] [PubMed]
- Grischke J, Eberhard J, Stiesch M. Antimicrobial dental implant functionalization strategies—A systematic review. Dent Mater J 2016;35:545-58. [Crossref] [PubMed]
- Diefenbeck M, Shrader C, Gras F, et al. Gentamycin coating of plasma chemical oxidized titanium alloy prevents implant related osteomyelitis in rats. Biomaterials 2016;101:156-64. [Crossref] [PubMed]
- Drago L, Boot W, Dimas K, et al. Does implant coating with antibacterial-loaded hydrogel reduce bacterial colonization and biofilm formation in vitro? Clin Orthop Relat Res 2014;472:3311-23. [Crossref] [PubMed]
- Fröjd V, Linderbäck P, Wennerberg A, et al. Effect of nanoporous TiO2 coating and anodized Ca2+ modification of titanium surfaces on early microbial biofilm formation. BMC Oral Health 2011;11:8. [Crossref] [PubMed]
- Visai L, Rimondini L, Giordano C, et al. Electrochemical surface modification of titanium for implant abutments can affect oral bacteria contamination. J Appl Biomater Biomech 2008;6:170-7. [PubMed]
- Xing R, Witsø IL, Jugowiec D, et al. Antibacterial effect of doxycycline-coated dental abutment surfaces. Biomed Mater 2015;10:055003. [Crossref] [PubMed]
- Cardoso M, Sangalli J, Koga-Ito CY, et al. Abutment Coating With Diamond-Like Carbon Films to Reduce Implant-Abutment Bacterial Leakage. J Periodontol 2016;87:168-74. [Crossref] [PubMed]
- Kheur S, Singh N, Bodas D, et al. Nanoscale silver depositions inhibit microbial colonization and improve biocompatibility of titanium abutments. Colloids Surf B Biointerfaces 2017;159:151-8. [Crossref] [PubMed]
- Huacho PMM, Nogueira MNM, Basso FG, et al. Analyses of Biofilm on Implant Abutment Surfaces Coating with Diamond-Like Carbon and Biocompatibility. Braz Dent J 2017;28:317-23. [Crossref] [PubMed]
- Brunello G, Brun P, Gardin C, et al. Biocompatibility and antibacterial properties of zirconium nitride coating on titanium abutments: An in vitro study. PLoS One 2018;13:e0199591. [Crossref] [PubMed]
- Odatsu T, Kuroshima S, Sato M, et al. Antibacterial Properties of Nano-Ag Coating on Healing Abutment: An In Vitro and Clinical Study. Antibiotics (Basel) 2020;9:347. [Crossref] [PubMed]
- Almohandes A, Abrahamsson I, Dahlén G, et al. Effect of biofilm formation on implant abutments with an anti-bacterial coating: A pre-clinical in vivo study. Clin Oral Implants Res 2021;32:756-66. [Crossref] [PubMed]
- Mukaddam K, Astasov-Frauenhoffer M, Fasler-Kan E, et al. Effect of a Nanostructured Titanium Surface on Gingival Cell Adhesion, Viability and Properties against P. gingivalis. Materials (Basel) 2021;14:7686. [Crossref] [PubMed]
- Javali MA, AlQahtani NA, Ahmad I, et al. Antimicrobial photodynamic therapy (light source; methylene blue; titanium dioxide): Bactericidal effects analysis on oral plaque bacteria: An in vitro study. Niger J Clin Pract 2019;22:1654-61. [Crossref] [PubMed]
- Zhang XM, Li Y, Gu YX, et al. Ta-Coated Titanium Surface With Superior Bacteriostasis And Osseointegration. Int J Nanomedicine 2019;14:8693-706. [Crossref] [PubMed]
- Zhou J, Wang X, Zhao L. Antibacterial, angiogenic, and osteogenic activities of Ca, P, Co, F, and Sr compound doped titania coatings with different Sr content. Sci Rep 2019;9:14203. [Crossref] [PubMed]
- Ding L, Zhang P, Wang X, et al. Effect of doxycycline-treated hydroxyapatite surface on bone apposition: A histomophometric study in murine maxillae. Dent Mater J 2018;37:130-8. [Crossref] [PubMed]
- Nie B, Ao H, Long T, et al. Immobilizing bacteria on titanium for prophylaxis of infection and improving osteoconductivity: an in vivo study. Colloids Surf B Biointerfaces 2017;150:183-91. [Crossref] [PubMed]
- Sterzenbach T, Helbig R, Hannig C, et al. Bioadhesion in the oral cavity and approaches for biofilm management by surface modifications. Clin Oral Investig 2020;24:4237-60. [Crossref] [PubMed]
- Elter C, Heuer W, Demling A, et al. Comparative analysis of biofilm formation on dental implant abutments with respect to supra- and subgingival areas: polytetrafluoroethylene versus titanium. Int J Prosthodont 2011;24:373-5. [PubMed]
- López-Valverde N, Macedo-de-Sousa B, López-Valverde A, et al. Effectiveness of Antibacterial Surfaces in Osseointegration of Titanium Dental Implants: A Systematic Review. Antibiotics (Basel) 2021;10:360. [Crossref] [PubMed]
- Lee SW, Phillips KS, Gu H, et al. How microbes read the map: Effects of implant topography on bacterial adhesion and biofilm formation. Biomaterials 2021;268:120595. [Crossref] [PubMed]
- Garaicoa JL, Bates AM, Avila-Ortiz G, et al. Antimicrobial Prosthetic Surfaces in the Oral Cavity-A Perspective on Creative Approaches. Microorganisms 2020;8:1247. [Crossref] [PubMed]
- Dijksteel GS, Ulrich MMW, Middelkoop E, et al. Review: Lessons Learned From Clinical Trials Using Antimicrobial Peptides (AMPs). Front Microbiol 2021;12:616979. [Crossref] [PubMed]
- Bronk JK, Russell BH, Rivera JJ, et al. A multifunctional streptococcal collagen-mimetic protein coating prevents bacterial adhesion and promotes osteoid formation on titanium. Acta Biomater 2014;10:3354-62. [Crossref] [PubMed]
- Zhou Z, Shi Q, Wang J, et al. The unfavorable role of titanium particles released from dental implants. Nanotheranostics 2021;5:321-32. [Crossref] [PubMed]
Cite this article as: Jensen OT, Weiss E, Tarnow D. Antimicrobial surface treatment of titanium dental implants: a narrative review between 2011 and 2021. Front Oral Maxillofac Med 2023;5:25.

