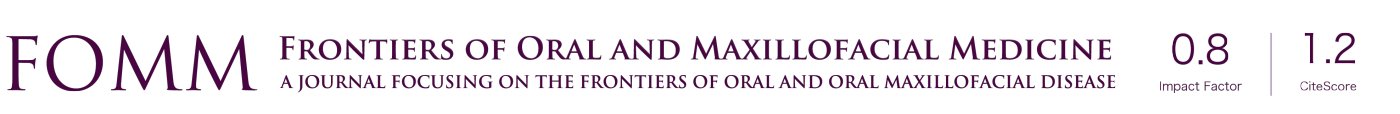Indications for replacement after alloplastic temporomandibular joint device failure: a narrative review
Introduction
There are many established and emerging alloplastic temporomandibular joint replacement (TMJR) systems on the international market with a varying degree of data available on long-term outcomes (1-3). A recently published survey of surgeons with high volume TMJR practice noted incidences of revision and replacement of 3% and 4.9% respectively. The most common reasons for revision and replacement were identified as being heterotopic ossification (27.5%) and infection (21.1%) respectively (4). This finding was echoed in a systematic review and meta-analysis by Bach et al. (5) who identified heterotopic bone formation as being the most common cause for TMJR revision or replacement (1.19/100 prosthesis-years), with surgical site infection being the second commonest (0.34/100 prosthesis-years). Reassuringly, in this review of modern approved systems (TMJ Concepts and Zimmer Biomet) reoperation rates for fixation/component loosening and malocclusion (suggesting faulty operative technique) was exceedingly uncommon (0.14/100 prosthesis-years and 0.06/100 prosthesis-years respectively) (5). All systems will develop wear under functional loading and development of an alloplastic system that never fails is unlikely.
The purposes of this paper are to present a narrative review examining the reasons for failure however, what can be done to mitigate against them and remedial action that can be taken when failure does occasionally occur. We present this article in accordance with the Narrative Review reporting checklist (available at https://fomm.amegroups.com/article/view/10.21037/fomm-22-6/rc).
Methods
A comprehensive literature search was undertaken in February 2022 using the electronic systems PubMed, Cochrane Library, ScienceDirect and Embase. Search terms were free text (MeSH terms were found to be overly restrictive): “TMJ”; “temporomandibular joint”; “TMJR”; “joint replacement”; “revision”; “explanted”; “removed”; and “failure”. Searches were restricted to articles published between 2000 and 2022 in English language journals. Full length articles returned in the search were reviewed in the preparation of this narrative review which was supplemented with discussion from the wider literature where warranted for readability. The PubMed search strategy summary is given in Table 1 and we included all articles returned that examined potential reasons for TMJR failure resulting in revision or replacement surgery. The remaining electronic systems were then used with the same search terms, excluding all duplicate or irrelevant articles. In the preparation of this article, we followed the categories in the Scale for the Assessment of Narrative Review Articles (SANRA) (6).
Table 1
| Items | Specification |
|---|---|
| Date of search | 27th April 2022 |
| Databases and other sources searched | PubMed, Cochrane Library, ScienceDirect, Embase |
| Search terms used | ((TMJ[Title/Abstract]) AND ((TMJR[Title/Abstract]) OR (joint replacement[Title/Abstract])) AND ((revision[Title/Abstract]) OR (failure[Title/Abstract]) OR (explanted[Title/Abstract]) OR (removed[Title/Abstract)) AND ("2000/01/01"[Date - Publication]: "3000"[Date - Publication])) |
| Timeframe | 2000–2022 |
| Inclusion and exclusion criteria | Published articles examining causes of TMJR failure resulting in revision or replacement surgery |
| Selection process | Full text articles reviewed by the authors for all search results returned with the above terms |
TMJR, temporomandibular joint replacement.
A brief history of failed of devices
It is fair to say that TMJR has undergone a checkered history with a variety of incarnations over the years. The forerunners were interpositional materials beginning with John Carnochan’s wooden spacer for gap arthroplasty surgery in 1840 through a range of other materials including gold, ivory, tantalum, stainless steel, silastic and Proplast-Teflon™ (Vitek, Houston, TX, USA). Hemiarthroplasties with condylar components only were developed in parallel beginning with rubber in the 19th century, with cobalt-chromium (Co-Cr) alloy first making an appearance in 1964 (although an interpositional Vitallium® plate preceded this in 1951) and titanium in 1976. Total joint replacements included the early Christensen devices in 1965 including polymethylmethacrylate (PMMA), passing through the use of Teflon™ (polytetrafluoroethylene, PTFE) and Proplast® by Vitek-Kent in 1983, until ultrahigh molecular weight polyethylene (UHMWPE) first appeared in 1986 (7).
For a device to be successful it has been identified that key considerations are biocompatibility, favourable wear characteristics, custom fit, good stability and corrosion resistance among other properties (8). Failures of devices throughout history highlighted that materials needed to be strong enough to prevent micromotion but adopt an elastic modulus mimicking bone to prevent bone resorption and failure of osseointegration.
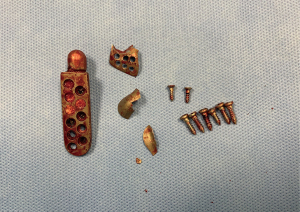
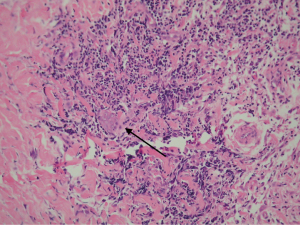
Foreign body giant cell reactions (FBGCR) were particularly prevalent in response to materials such as Teflon™ and polydimethylsiloxane silicone or Silastic™ (Dow Corlington, Midland MI), leading to catastrophic results and a widespread professional backlash to devices when regulatory body approval was withdrawn (as in the case of FDA approval for the Vitek disc prosthesis) (7). Figures 1-3 demonstrate a failed Christensen device, a not uncommon complication due to micromotion with corrosion and fatigue of the fossa leading to eventual fracturing, the downfall of a metal-on-metal design in the TMJ. These devices were actually used to replace UHMWPE containing devices in the Vitek VK II system, again let down by the potential for FBGCR due to the Proplast® II lining of the components (9). The Christensen devices themselves were then subsequently replaced by TMJ Concepts devices due to unfavourable reactions in a number of cases resulting in their explantation. Figure 4 demonstrates histological confirmation of FBGCR surrounding a failed Christensen device. The reactions were conjectured to be due to either metal allergy, point contact wear phenomenon, micromovement or lymphocyte-mediate immunological reaction (10). Figure 5 shows the device following removal.
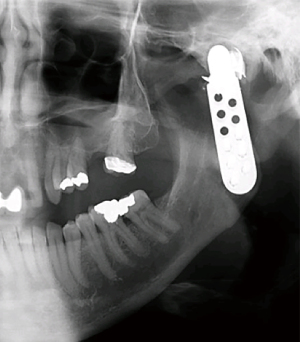
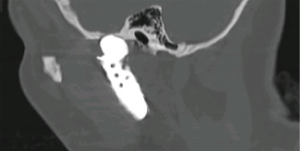
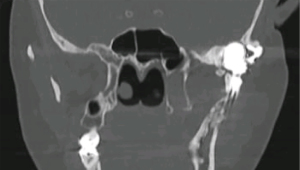
The formation of wear debris in many devices, leading to metallosis, FBGCR and allergic and inflammatory reactions has given pause for thought in ensuring systems are fit for purpose in terms of both in vitro testing and careful collection of post-operative outcomes over the longer term. Such robust and transparent data reporting is the hallmark of modern TMJR surgery.
Reasons for failure requiring revision surgery or removal
The PubMed search conducted (see Table 1) returned 33 articles in total. One article was a duplication of outcomes from a single patient cohort and was excluded. A further excluded article was a prospective cohort study from a single centre which had no failures resulting in revision or replacement. From the remaining articles, 22 were not relevant to the aims of this paper, leaving 9 papers for inclusion. Additional searches in Embase, ScienceDirect and Cochrane Library increased the number of included articles to 13. Revision surgery rates ranged from 1.6% to 11.2% with cited reasons for failure including heterotopic bone formation, infection, incorrect placement, hardware failure, fibrous ankylosis, hypersensitivity and dislocation. These papers are summarized in Table 2.
Table 2
| Study | Year published | Study design | Number of cases included | System used | Failure rate | Causes of failure identified | Additional comments |
|---|---|---|---|---|---|---|---|
| Amarista et al. (4) | 2020 | Clinician survey | 4,638 procedures | Various | 3% revision rate, 4.9% replacement rate | Heterotopic bone, infection | Revision success 86.7%, replacement success 94.6% |
| Granquist et al. (11) | 2020 | Prospective cohort study | 499 joints, 319 patients | Biomet (custom) | 11.2% requiring “subsequent surgical intervention” (4.2% removal) | Fibrous ankylosis, heterotopic bone formation, infection, hardware failure, dislocation, incorrect placement, foreign body reaction, hardware failure | 16.1% of re-operated patients required a further procedure (and 11.1% of these required a third |
| Machoň et al. (12) | 2020 | Prospective cohort study | 62 joints, 45 patients | Biomet (stock) | 1.6% (n=1) requiring revision | Infection | |
| Bach et al. (5) | 2022 | Systematic review and meta-analysis | 2,247 joints | 1,350 stock (Biomet), 897 custom (Biomet and TMJ Concepts) | 1.19/100 prosthesis-years revision rate | Heterotopic bone formation, infection, component loosening and/or malposition, hypersensitivity, malocclusion, dislocation | |
| Lee et al. (13) | 2021 | National database query | 392 adverse events | Not specified | N/A | Infection, heterotopic bone formation, poor operative fit | |
| Kerwell et al. (14) | 2016 | Examination of explanted TMJR devices | 31 TMJRs, 28 failed, 3 controls | Not specified | N/A | N/A | Damage included pitting corrosion, hard phases, surface depression, bi-directional scratches |
| Gruber et al. (15) | 2015 | Retrospective cohort study | 84 joints, 58 patients | TMJ Concepts | 2.4% (n=2) removal | Infection | |
| Schuurhuis et al. (16) | 2012 | Prospective cohort study | 14 joints, 8 patients | Groningen | 7.1% (n=1) removal | Not clearly specified | The authors stated that “the patient could not endure the prosthesis” |
| Aagaard and Thygesen (17) | 2014 | Prospective cohort study | 81 joints, 61 patients | Biomet (custom) | 2.5% (n=2) | Infection, hypersensitivity | |
| Gakhal et al. (18) | 2020 | Retrospective cohort study | 26 joints, 20 patients | Christensen and TMJ Concepts | N/A | Infection, allergy, surface wear, reankylosis, fractured prosthesis | Paper specifically focused on a cohort of revision replacements |
| Gonzalez-Perez et al. (19) | 2016 | Prospective cohort study | 68 joints, 52 patients | Biomet (stock) | 2.9% (n=2) removed | Malocclusion, component loosening | |
| Park et al. (20) | 2004 | Retrospective cohort study | 108 joints, 84 patients | Christensen fossa-eminence hemijoint | 8.0% (n=9) removed | Pain, loosening of components, heterotopic bone formation | |
| Giannakopolous et al. (21) | 2012 | Prospective cohort study | 442 joints, 288 patients | Biomet (stock) | 3.2% (n=14) removed | Heterotopic bone, infection |
TMJR, temporomandibular joint replacement.
The role of prosthetic joint infection (PJI) in TMJ TJR failure
Infection is fortunately an uncommon complication of TMJR with one large cohort study reporting an incidence rate of 1.51% for PJIs at a mean of six months post-operatively (22). PJIs may be divided as early and late, distinguished by time of onset before or after three months post-operatively respectively, according to the classification devised by Fitzgerald et al. (23). Acute or early infections are largely nosocomial and acquired at the time of implantation of the TMJR most commonly due to skin commensals such as Staphylococcus epidermidis and Staphylococcus aureus. Late infections may be further subdivided as late deep infections (appearing between three months and two year post-operatively) and late haematological infections (appearing beyond two years) (24,25). Amarista et al. highlighted infection being the commonest reason for explantation of TMJRs and replacement in a survey of surgeons spanning 4,638 procedures (4). Cohorts from Machoň et al. (12), Gruber et al. (15) and Gakhal et al. (18) would seem to echo this, and Lee et al. (13) conducted a National Inpatient Sample query in the United States to demonstrate that infection was among the most common adverse events in TMJR surgery.
The concern with all infections is the formation of biofilms on the prosthesis surface through coalescing exo-polysaccharides, offering protection from host immune defenses and antibiotic permeation (26). Diagnosis of PJI of TMJR devices can be challenging as the clinical presentation may be subtle with just pain and diffuse swelling, but not necessarily classical hallmarks of infection such as erythema, fever and/or cutaneous fistula formation. Diagnosis of early infections can be guided by elevated inflammatory markers (26). In late PJIs of TMJRs, inflammatory markers show poor correlation and joint aspiration for white cell counts and culture as well as radiolabelled leukocyte imaging should be considered alongside standard plain film radiographs and/or cross-sectional CT. Synovial fluid levels of components such as neutrophil gelatinase-associated lipocalin and α-defensins have also been highlighted as potential biomarkers of PJI in wider orthopaedic literature (26).
The key is prevention of infection in the first instance. Clearly risks of infection cannot be completely obviated and patient factors may be involved including immunosuppression (e.g., concurrent biologic treatments and disease modifying anti-rheumatic drugs or DMARDs), diabetes mellitus, the multiply operated patient, smoking, poor nutritional status and any pre-existing remote and local site infections (24,25).
Once PJI is established it is important to work in close conjunction with colleagues in Microbiology. Ideally antimicrobials should be started only after joint aspiration in late PJI, with a suspension in empirical or targeted therapy prior to surgery to enable culture of peri-prosthetic tissue. It has been the authors’ practice to place spacers fabricated with antibiotic-impregnated polymethylmethacrylate (PMMA) in between two stage surgery for removal and replacement of infected TMJRs, as well as continuing long-term antibiotic therapy refined by any positive culture results, involving the outpatient parenteral antibiotic therapy (OPAT) team where warranted. Novel therapies may include expanded ranges of broad-spectrum antimicrobials, antibiotics with biofilm bactericidal concentrations and small molecule delivery systems (27).
Biocompatability
Biocompatability is a key consideration in material selection for any prosthetic implantable device and the concept refers to the ability of the material to be in contact with native tissues without eliciting any adverse reaction, including any degradation products produced through wear (28). Failed devices as a result of biocompatibility issues may be a result of one or a combination of mechanisms including FBGCRs and material hypersensitivity.
The importance of biocompatibility in material selection is perhaps best exemplified by the now discontinued interpositional disc implants (IDIs) fabricated from Proplast-Teflon™ (Vitek, Houston, TX, USA) and Silastic™ (Dow Corning, Midland, MI, USA). Functional loading of these materials showed favourable wear characteristics in vitro but the FBGCR to particulate debris generated in vivo was marked and many patients suffered severe pain following implantation, as well as advancing degenerative changes with bone resorption (29). This, despite warnings issued in the orthopaedic literature by Charnley as early as 1963, was also seen beyond IDIs in TMJR systems issued as the Vitek-Kent, featuring the use of a Proplast® and high-density polytetrofluoroethylene (Teflon™) fossa (27). Extracellular particles from wear in such devices have been demonstrated as becoming coated with proteins inducing chemotaxis and phagocytosis by macrophages. Enzymatic activation is triggered and eventual exocytosis of the particulate matter following failed attempts to degrade the material. This then triggers further chemotaxis setting up a “vicious cycle” and resultant injury to surrounding tissue with resultant device failure (30).
Similarly, silicone rubber or polydimethylsiloxane (Silastic™) devices demonstrate particulate silicone debris with FBGCRs in vivo leading to “silicone synovitis” with osteoclast upregulation and the similar progressive loosening of implants and failure (30). Polymethylmethacrylate (PMMA) has also found application early in the history of prosthetic joint replacement with the Judet hip implant and continued to be used in leveling of the articular surfaces of the ultra-high-molecular-weight polyethylene (UHMWPE) fossa component in the Biomet/Lorenz TMJ TJR system until fairly recently. Again, this demonstrates wear particles and osteolysis, with fragmentation under functional loading and its use has been discontinued in this manner (28,30,31).
Design faults and material wear
We have previously highlighted a number of emerging TMJ TJR systems around the world that use a variety of materials, designs and manufacturing techniques but many lack clinical data beyond isolated case reports or case series and others have not gone as far as even reporting pre-clinical laboratory data (1). Design flaws such as a failure to include the entire length of the lateral mandibular ramus for the condyle/ramus component place undue strain with resultant micromotion and eventual failure based on data extrapolated from finite element analysis (FEA) research (1,31,32). Similarly design features such as cantilevers and/or multiple moving parts have been described by Mercuri as “violating the mechanical and immunobiological principle of this joint” and therefore likely to fail (30).
Metal-on-metal coupling is liable to develop particulate debris and tribocorrosion (a combination of mechanical wear and electrochemical reactions). In the latter situation, the loss of a protective oxide layer renders articular surfaces prone to erosion and fretting of surfaces, with the metal ions generated shown to promote peri-implant tissue reactions. Metal-on-metal systems and the use of highly corrosive materials such as stainless steel are therefore doomed to fail early (14,33). As noted, metal component allergy, point contact wear, micro-movement and lymphocyte-mediated immunological reaction to prosthetic material were cited as potential reasons for foreign body-type responses seen in patients following TMJR across three well established surgeons’ practice in the United Kingdom using the Christensen TMJ System (or Nexus CMF TMJ Total Joint Prosthesis), which subsequently fell out of favour (10). Christensen systems featured fossa and condylar head components fabricated from cast cobalt-chromium-molybdenum (Co-Cr-Mo) alloy and following the poor outcomes reported the United States Food and Drug Administration (FDA) withdrew approval. This in turn led to establishing a national database to track outcomes of TMJ TJR across the country (34). In our literature search, the retrospective study of Park et al. (20) examining 108 Christensen TMJRs in 84 patients showed a removal rate of 8.0% with reasons appearing to deviate from other cohorts to more prominently feature component loosening. Clearly design is of paramount importance as even in stock Zimmer Biomet TMJR cohorts explantation rates were lower (19).
Polyether ether ketone (PEEK) has also been suggested as an alternative material for use in TMJ TJR systems with a small case series reported on by a Brazilian group, as well as in vitro testing reported more recently from China (35,36). We have previously raised concerns of unfavourable wear characteristics from the orthopaedic literature when compared with UHMWPE (1).
Surgical faults
It is important that the TMJR surgeon is undertaking sufficiently high volumes of tertiary referral work that skills are maintained and the best possible surgical outcomes achieved. Custom devices demonstrate a better adaptation when compared with stock TMJRs, ultimately resulting in less micromotion and potential for component loosening and mechanical failure (37). Optimum fit is to some degree dependent upon surgeon skill however, and potential mistakes may include failing to clear soft tissues sufficiently from the fossa prior to seating the fossa component (leading to interposition of soft tissue and “rocking”) and inadequate clearance at the low condylectomy cut (and/or beveling) to enable to the ramus component to seat passively. The use of custom cutting guides may help with this (and minimize undue handling of the implantable components), but there is no substitute for experience to minimize operating times and the potential for surgical errors.
Granquist et al. (11) returned a figure of 11.2% of their custom Zimmer Biomet TMJRs requiring a “subsequent surgical intervention” (SSI), of whom 16.1% required a second SSI and of these, 11.1% required a third. In their cohort, removal of the TMJR was required at an initial SSI in 4.2% with reasons spanning infection, dislocation, heterotopic bone formation, hardware failure and fibrous ankylosis, but importantly incorrect positioning of the prosthesis was also given as a reason for revision and/or removal.
Timely decision-making is also key, as poorer outcomes are seen in the multiply operated patient, particularly one with prior exposure to failed materials (28). As such, ensuring candidates for TMJR are seen early in the decision-making process is integral to surgical success.
A further key step in planning is consideration of the soft tissue envelope. Cross-sectional imaging of the maxillofacial hard tissues can provide a false sense of security regarding the chances of success in achieving prosthetic rehabilitation of the TMJ. This is particularly true in patients with restrictive soft tissues as a result of causes including long-standing ankylosis, hemifacial microsomia, Pierre-Robin sequence and radiotherapy-induced fibrosis among others. In such cases the tight soft tissue envelope may preclude consideration of the option of alloplastic replacement, but techniques such as transport distraction osteogenesis and the Matthews Device may overcome this (38).
Patient factors
Immunosuppression due to any underlying aetiology can be presupposed to contribute to the potential for TMJR failure. This is a key concern, as patients with inflammatory arthritides in particular may be on DMARDs, biologic agents and/or systemic corticosteroids. In addition, by definition dietary intake in the run up to surgery may have been poor and malnutrition may be a further contributor to an impaired immune response. In patients with inflammatory arthritides in particular, it is essential to liaise closely with the extended team involved in their care. Collaboration with rheumatologists will inform peri-operative decisions such as the prospective impact of medical therapies on symptom control and disease progression, as well as the timing (and need) for cessation of medications such as monoclonal antibodies.
Heterotopic bone formation is another patient factor that dictates the need for revision or replacement surgery in failed TMJRs. This was highlighted as the commonest cause for revision surgery in the survey conducted by Amarista et al. (4) at 27.5% with an overall success rate for revision surgery quoted at 86.7%. In the systematic review and meta-analysis by Bach et al. (5) spanning 2,247 devices, heterotopic bone formation was again the most common reason for revision surgery, which they calculated to be 1.19 per 100 prosthesis-years. Similarly in a prospective cohort study by Giannakopolous et al. (21), a 3.2% explantation rate was largely contributed to by cases of heterotopic bone formation.
One of the patient factors that has received most attention to date however is hypersensitivity to implant components. It is noted in particular that the incidence of metal sensitivity is higher in patients with painful failing alloplastic implants when compared with both the general population and patients with successful similar implants (39).
The first key consideration is that the implant surfaces themselves are not at fault, rather the debris as a result of mechanical wear. Such debris results in metal ions acting as haptens to join with endogenous proteins in being engulfed by antigen-presenting cells, triggering the immune response. As such, hypersenstitivity tends to be cell-mediated immune responses from conditioned lymphocytes i.e., a type IV or delayed-type hypersensitivity reaction (40). Nickel can also activate an immune response from non-classical means, acting as a superantigen to directly stimulate T cell receptors (41).
It is the authors’ practice to ask for skin testing (patch testing) for allergy to the components of the intended TMJR prosthesis. There is variation in practice however, with some UK surgeons routinely offering the all titanium alloy TMJ Concepts ramus component and others merely querying a history of contact allergy to “costume jewelry” with the patient (41,42). Skin testing is a poor surrogate however, with allergenic potential of metals in the dermis with Langerhans’ cells as the antigen-presenting cells likely to be different to the peri-prosthetic environment. There is also the concern of inducing hypersensitivity in an individual, as well as the subjective and non-standardized nature of the test (40,43).
Lymphocyte transformation testing (LTT) is argued to be better suited to testing the potential for implant-related sensitivity than dermal patch testing, with quantitative results, dose-dependent reactivity and metal-protein complexes similar to those seen in vivo being cited as potential strengths of the test. Dermal patch testing would appear to correlate poorly with more sensitive tests such as LTT and cytokine analysis and the accuracy is regarded as poor by most orthopaedic surgeons (44). Availability of LTT is limited however, leading to Dodd and Begley (45) highlighting the alternative of Memory Lymphocyte Immunostimulation Assay (MELISA®), cited as an optimized and more accurate variant of LTT available commercially.
Metal sensitivity is linked to failure of devices and associated pain, arguably being a substantial contributor to TMJR failure. Symptoms related to metal device allergy can be elusive and difficult to positively attribute to a cause (46). It is important to be wary about attributing pain post-TMJR to hypersensitivity or infection. To do so would almost certainly lower the threshold for re-operation unduly, as post-operative pain does not automatically imply TMJR failure. Indeed, in two of the papers returned in our literature search the causes for explantation were uncertain. Schuurhuis and colleagues (16) stated simply that “the patient could not endure the prosthesis in her body”, whilst Aagaard and Thygesen (17) had a case of presumed PJI and another of hypersensitivity, although neither demonstrated concrete findings on objective testing methods.
Future considerations
Many of the devices we previously reviewed were products of the increasingly widespread availability of virtual planning and selective laser melting (SLM) and direct metal laser sintering (DMLS) 3D printing methods as cost effective in-house manufacturing alternatives. Evidence has demonstrated however that such systems are liable to cause high porosity, residual stress, cracking, warping and surface roughness not seen in devices with better longevity such as TMJ Concepts custom prostheses (1,47).
Clearly computer-assisted design/computer-assisted manufacture (CAD/CAM) is shaping the development of systems with an emphasis on custom TMJRs dominating the market. New coatings are emerging (e.g., β-titanium and alumina-toughened zirconia) and others will no doubt be forthcoming (7).
Revision surgery involves additional cost, time and in particular considerable additional morbidity to the patient. Explantation of failed TMJRs will mandate an intermediate stage with no device in situ prior to establishing safety for re-implantation of a replacement TMJR. This potentially commits patients to a period of intermaxillary fixation (IMF), reduced oral intake and impaired quality of life. Adding to this the observation that the multiply operated patient is at a higher risk of facial nerve injury, clearly the emphasis should be on achieving the best possible outcome first time around (48).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Louis G. Mercuri) for the series “Indications for Alloplastic TMJ Replacement in Maxillofacial Surgery—an evidence-based review of the literature” published in Frontiers of Oral and Maxillofacial Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://fomm.amegroups.com/article/view/10.21037/fomm-22-6/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://fomm.amegroups.com/article/view/10.21037/fomm-22-6/coif). The series “Indications for Alloplastic TMJ Replacement in Maxillofacial Surgery—an evidence-based review of the literature” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Elledge R, Mercuri LG, Attard A, et al. Review of emerging temporomandibular joint total joint replacement systems. Br J Oral Maxillofac Surg 2019;57:722-8. [Crossref] [PubMed]
- Johnson NR, Roberts MJ, Doi A, et al. Total temporomandibular joint replacement prostheses: a systematic review and bias adjusted metanalysis. Int J Oral Maxillofac Surg 2017;46:86-92. [Crossref] [PubMed]
- Wolford LM, Mercuri LG, Schneiderman ED, et al. Twenty year follow-up study on a patient-fitted temporomandibular joint prosthesis: the Techmedica/TMJ Concepts device. J Oral Maxillofac Surg 2015;73:952-60. [Crossref] [PubMed]
- Amarista FJ, Mercuri LG, Perez D. Temporomandibular Joint Prosthesis Revision and/or Replacement Survey and Review of the Literature. J Oral Maxillofac Surg 2020;78:1692-703. [Crossref] [PubMed]
- Bach E, Sigaux N, Fauvernier M, et al. Reasons for failure of total temporomandibular joint replacement: a systematic review and meta-analysis. Int J Oral Maxillofac Surg 2022;51:1059-68. [Crossref] [PubMed]
- Baethge C, Goldbeck-Wood S, Mertens S. SANRA-a scale for the quality assessment of narrative review articles. Res Integr Peer Rev 2019;4:5. [Crossref] [PubMed]
- De Meurechy N, Mommaerts MY. Alloplastic temporomandibular joint replacement systems: a systematic review of their history. Int J Oral Maxillofac Surg 2018;47:743-54. [Crossref] [PubMed]
- Wolford LM, Mehra P. Custom-made total joint prostheses for temporomandibular joint reconstruction. Proc (Bayl Univ Med Cent) 2000;13:135-8. [Crossref] [PubMed]
- Speculand B, Hensher R, Powell D. Total prosthetic replacement of the TMJ: experience with two systems 1988-1997. Br J Oral Maxillofac Surg 2000;38:360-9. [Crossref] [PubMed]
- Sidebottom AJ, Speculand B, Hensher R. Foreign body response around total prosthetic metal-on-metal replacements of the temporomandibular joint in the UK. Br J Oral Maxillofac Surg 2008;46:288-92. [Crossref] [PubMed]
- Granquist EJ, Bouloux G, Dattilo D, et al. Outcomes and Survivorship of Biomet Microfixation Total Joint Replacement System: Results From an FDA Postmarket Study. J Oral Maxillofac Surg 2020;78:1499-508. [Crossref] [PubMed]
- Machoň V, Levorová J, Hirjak D, et al. Evaluation of complications following stock replacement of the temporomandibular joint performed between the years 2006 and 2015: a retrospective study. Oral Maxillofac Surg 2020;24:373-9. [Crossref] [PubMed]
- Lee KC, Chintalapudi N, Halepas S, et al. The healthcare burden and associated adverse events from total alloplastic temporomandibular joint replacement: a national United States perspective. Int J Oral Maxillofac Surg 2021;50:236-41. [Crossref] [PubMed]
- Kerwell S, Alfaro M, Pourzal R, et al. Examination of failed retrieved temporomandibular joint (TMJ) implants. Acta Biomater 2016;32:324-35. [Crossref] [PubMed]
- Gruber EA, McCullough J, Sidebottom AJ. Medium-term outcomes and complications after total replacement of the temporomandibular joint. Prospective outcome analysis after 3 and 5 years. Br J Oral Maxillofac Surg 2015;53:412-5. [Crossref] [PubMed]
- Schuurhuis JM, Dijkstra PU, Stegenga B, et al. Groningen temporomandibular total joint prosthesis: an 8-year longitudinal follow-up on function and pain. J Craniomaxillofac Surg 2012;40:815-20. [Crossref] [PubMed]
- Aagaard E, Thygesen T. A prospective, single-centre study on patient outcomes following temporomandibular joint replacement using a custom-made Biomet TMJ prosthesis. Int J Oral Maxillofac Surg 2014;43:1229-35. [Crossref] [PubMed]
- Gakhal MK, Gupta B, Sidebottom AJ. Analysis of outcomes after revision replacement of failed total temporomandibular joint prosthesis. Br J Oral Maxillofac Surg 2020;58:220-4. [Crossref] [PubMed]
- Gonzalez-Perez LM, Fakih-Gomez N, Gonzalez-Perez-Somarriba B, et al. Two-year prospective study of outcomes following total temporomandibular joint replacement. Int J Oral Maxillofac Surg 2016;45:78-84. [Crossref] [PubMed]
- Park J, Keller EE, Reid KI. Surgical management of advanced degenerative arthritis of temporomandibular joint with metal fossa-eminence hemijoint replacement prosthesis: an 8-year retrospective pilot study. J Oral Maxillofac Surg 2004;62:320-8. [Crossref] [PubMed]
- Giannakopolous HE, Sinn DP, Quinn PD. Biomet Microfixation temporomandibular joinr replacement system: a 3-year follow-up study of patients treated during 1995 to 2005. J Oral Maxillofac Surg 2012;70:787-94. [Crossref] [PubMed]
- Mercuri LG, Psutka D. Peri-operative, post-operative and prophylactic use of antibiotics in alloplastic total temporomandibular joint replacement surgery: a survey and preliminary guidelines. J Oral Maxillofac Surg 2011;69:2106-11. [Crossref] [PubMed]
- Fitzgerald RH Jr, Nolan DR, Ilstrup DM. Deep wound sepsis following total hip arthroplasty. J Bone Joint Surg 1977;59:847-55. [Crossref] [PubMed]
- Mercuri LG. Complications associated with TMJ TJR: management and prevention. In: Mercuri LG. editor. Temporomandibular Joint Total Joint Replacement - TMJ TJR. Switzerland: Springer International Publishing, 2016.
- Mercuri LG. Prevention and detection of prosthetic temporomandibular joint infections-update. Int J Oral Maxillofac Surg 2019;48:217-24. [Crossref] [PubMed]
- Mercuri LG. Microbial biofilms - a potential source of alloplastic device failure. J Oral Maxillofac Surg 2006;64:1303-9. [Crossref] [PubMed]
- Ricciardi BF, Muthukrishnan G, Masters EA, et al. New developments and future challenges in prevention, diagnosis and treatment of prosthetic joint infection. J Orthop Res 2020;38:1423-35. [Crossref] [PubMed]
- De Meurechy N, Braem A, Mommaerts MY. Biomaterials in temporomandibular joint replacement: current status and future perspectives-a narrative review. Int J Oral Maxillofac Surg 2018;47:518-33. [Crossref] [PubMed]
- Mercuri LG, Giobbie-Hurder A. Long-term outcomes after total alloplastic temporomandibular joint reconstruction following exposure to failed materials. J Oral Maxillofac Surg 2004;62:1088-96. [Crossref] [PubMed]
- Mercuri LG. Management of Failing and Failed TMJ TJR Devices. In: Mercuri LG. editor. Temporomandibular Joint Total Joint Replacement - TMJ TJR. Switzerland: Springer International Publishing, 2016.
- d'Aubigne RM, Postel M. Functional results of hip arthroplasty with acrylic prostheses. J Bone Joint Surg 1954;36:451. [Crossref] [PubMed]
- Kashi A, Chowdhury AR, Saha S. Finite element analysis of a TMJ implant. J Dent Res 2010;89:241-5. [Crossref] [PubMed]
- Mathew MS, Kerwell S, Alfaro M, et al. Tribocorrosion and TMJ TJR devices. In: Mercuri LG. editor. Temporomandibular Joint Total Joint Replacement - TMJ TJR. Switzerland: Springer International Publishing, 2016.
- Elledge R, Attard A, Green J, et al. UK temporomandibular joint replacement database: a report on one-year outcomes. Br J Oral Maxillofac Surg 2017;55:927-31. [Crossref] [PubMed]
- Genovesi, W. A new concept and design for an alloplastic total TMJ prosthesis using PEEK LTI 20% BA. October 12, 2018. Available online: https://aaoms.confex.com/aaoms/am1810/meetingapp.cgi/Paper/13584 (last accessed January 1, 2022)
- Guo F, Huang S, Hu M, et al. Biomechanical evaluation of a customised 3D-printed polyetheretherketone condylar prosthesis. Exp Ther Med 2021;21:348. [Crossref] [PubMed]
- Mercuri LG. Alloplastic temporomandibular joint replacement: rationale for the use of custom devices. Int J Oral Maxillofac Surg 2012;41:1033-40. [Crossref] [PubMed]
- Kelly M, Bowen A, Murray DJ. Efficacy of temporomandibular joint arthroplasty and insertion of a Matthews device as treatment for ankylosis of the joint: a case series. Br J Oral Maxillofac Surg 2021;59:1113-9. [Crossref] [PubMed]
- Gabbay JS, Heller JB, Song YY, et al. Temporomandibular joint bony ankylosis: comparison of treatment with transport distraction osteogenesis or the matthews device arthroplasty. J Craniofac Surg 2006;17:516-22. [Crossref] [PubMed]
- Hallab N. Material hypersensitivity. In: Mercuri LG. editor. Temporomandibular Joint Total Joint Replacement - TMJ TJR. Switzerland: Springer International Publishing, 2016.
- Mercuri LG, Caicedo MS. Material Hypersensitivity and Alloplastic Temporomandibular Joint Replacement. J Oral Maxillofac Surg 2019;77:1371-6. [Crossref] [PubMed]
- Gawkrodger DJ. Nickel dermatitis: how much nickel is safe? Contact Dermatitis 1996;35:267-71. [Crossref] [PubMed]
- Munro-Ashman D, Miller AJ. Rejection of metal to metal prosthesis and skin sensitivity to cobalt. Contact Dermatitis 1976;2:65-7. [Crossref] [PubMed]
- Jacobs JJ. Clinical manifestations of metal allergy. Adverse reactions to byproducts of joint replacements (AAOS/ORSI). Presented at: Annual Meeting of the American Academy of Orthopaedic Surgery; San Francisco, CA. February 7-11, 2012.
- Dodd M, Begley A. The utility of MELISA® testing for metal allergy in patients requiring TMJ replacement. Br J Oral Maxillofac Surg 2019;57:e87. [Crossref]
- Teo Wendy ZW, Schalock PC. Hypersensitivity Reactions to Implanted Metal Devices: Facts and Fictions. J Investig Allergol Clin Immunol 2016;26:279-94. [Crossref] [PubMed]
- Megahed M, Mindt HW, N'Dri N, et al. Metal additive-manufacturing process and residual stress modelling. Integr Mater Manuf Innov 2016;5:61-93. [Crossref]
- Saeed NR, McLeod NMH. Predictive risk factors for facial nerve injury in temporomandibular joint replacement surgery. Br J Oral Maxillofac Surg 2021:S0266-4356(21)00164-9.
Cite this article as: Elledge ROC, Speculand B. Indications for replacement after alloplastic temporomandibular joint device failure: a narrative review. Front Oral Maxillofac Med 2023;5:33.