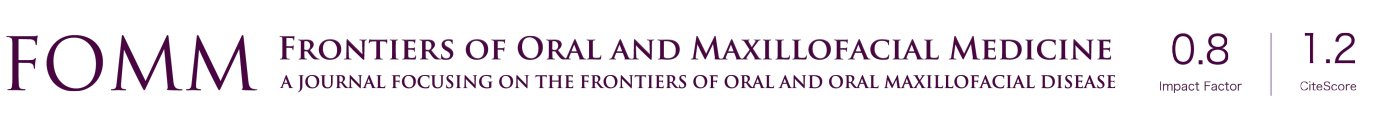Re-treatment of failed maxillary implant sites with severe bone loss from peri-implant disease: case series
Introduction
Dental implants have become a predictable and well-accepted module for the restoration of partially and fully edentulous patients with high survival rates ranging from 91.1% to 100% over a 10-year period (1). Nevertheless, with the growth in the use of dental implants by expanding groups of dental practitioners with a wide range of surgical and prosthodontic skills and experience, the rate of implants associated complications is rising (2).
The consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions has defined peri-implantitis as “a plaque-associated pathological condition occurring in tissues around dental implants, characterized by inflammation in the peri-implant mucosa and subsequent progressive loss of supporting bone” (3). It has been estimated that the average rate of peri-implantitis ranges between 15–22% (4-6), with a rate of 14.5% of moderate to severe peri-implantitis (7). It has also been shown that the incidence of peri-implantitis rises dramatically with time, with an average incidence at 5 and 10 years post-implantation of 0–3.4% and 10.7–47.2% respectively (8).
At some point in the progression of peri-implant disease leading to bone loss around dental implants, the decision has to be made to remove the implant(s) and to re-treat. However, the threshold amount of bone loss acceptable/not acceptable to clinicians and patients may vary (9,10). Also, the sustainable re-treatment probability of any given implant with significant bone loss from peri-implant disease is unpredictable (11).
When undertaking the process of re-treating a failed implant site following implant removal, with severe bone loss caused by peri-implant disease, it is crucial to keep in mind that the approach to such sites must be planned differently than the approach to an implant site with severe bone loss that has not yet undergone previous implantation (12-14). This is, among other reasons, due to residual inflammatory processes (15), compromised soft tissue (16), altered residual bone physiology (17), and reduced wound-healing potential at the re-treated site (18). Unfortunately, these and other factors have been shown to play a part in a reduced prognosis of replaced implants with lower success rates with every additional implant replacement attempt (19,20).
In the present manuscript, the re-treatment of failed implant sites will be presented and the key factors for re-treatment success will be discussed. In addition, a recommended workflow for the re-treatment of failed implant sites will be offered as a clinician’s guide.
The unifying theme of this study is that peri-implant disease can be ablative requiring extensive e surgery to correct but can be anticipated and preventable in well monitored patients who have incipient peri-implant disease. We present the following article in accordance with the PROCESS reporting checklist (available at https://fomm.amegroups.com/article/view/10.21037/fomm-21-111/rc).
Methods
We present the following two case reports correlated as a “case series” to elucidate retreatment aspects of ablative peri-implant disease. The patients were treated as a matter of course in two clinical practices, one in the United States and one in Israel. This was a retrospective study based on chart review. Patient permission for publication of pictures and data was obtained. A copy of the written consent is available for review by the editorial office of this journal. The setting was academic in Israel at Hebrew University in Jerusalem and in Denver, Colorado at a private practice setting for oral and maxillofacial surgery.
Patient participants in the study were healthy patients who had undergone implant treatment in private clinical practice but some years afterward developed severe peri-implant disease. There were no pre-interventive considerations as this study reports and discusses treatment of complications. Intervention was surgical removal, bone augmentation and reimplantation.
Peri-intervention considerations were limited to surgery as a pharmacological approach was not possible. Surgery was performed by two operators (M Alterman and OT Jensen). Quality control of the surgical procedures was based on oral maxillofacial surgery principles of surgery and attendant medical management. Post-operative care was according to basic principle of wound care and follow-up including antibiotic prophylaxis.
The manuscript presents a retrospective evaluation of two unique cases, without any interventional study, nor patient identifying data presented. Hence, the manuscript does not require an ethical approval. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Results
Two surgical procedures were completed including implant removal, debridement, antimicrobial medications, bone graft reconstruction including the use3d of biomimetic bone morphogenetic protein 2 (BMP-2) and reimplantation followed by dental restoration. The patient treated in the United States received a titanium mesh with BMP-2/collagen sponge with allogenic bone chips which uniquely demonstrated highly vascularized bone with excellent vitality for placement of dental implants. Outcome was favorable in both cases of retreatment with long term follow-up showing stable implants and bone graft persistence. No implants were lost subsequent to retreatment. There were no complications from treatment in the two cases reported.
Case presentation
Two clinical cases are presented in this manuscript, describing the steps, methods, and principles of the re-treatment procedure after implants failure. The first case describes a failed implant-supported segmental rehabilitation while the second focused on a failed implant-supported full-arch rehabilitation. In both cases, the various procedure stages are thoroughly described and discussed.
For the discussion, an electronic search was performed by PubMed and MEDLINE databases, using the combination of the following terms: “Implant failure”, “Implant removal”, “Peri-implantitis”, and “Retreatment”.
Case 1—failed implant-supported segmental rehabilitation
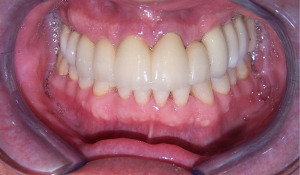
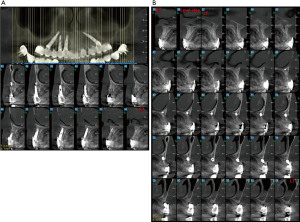
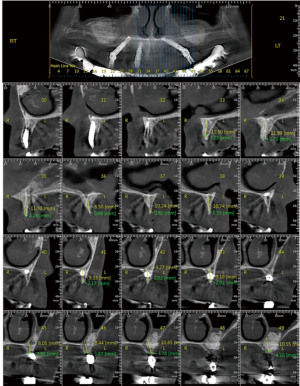
Complete maxillary arch dental extractions for periodontitis (Figure 1) were done in a 50-year-old healthy male patient, with the placement of 10 immediately placed dental implants later restored with three fixed ceramo-metal bridges. Three implants on the upper left side had been placed too close together (Figure 2) without platform bone switch and some years later developed significant bone loss from peri-implant disease. Bone had been stable on all other implants. Since the loss of bone was horizontal and an adjacent implant was not affected but in close proximity, it was decided to remove the three implants and let the defect site heal and reconstruct some months later (Figure 3). The idea was to eradicate the peri-implant disease, prevent it from spreading to adjacent implants rather than attempt implant decontamination and peri-implant grafting. The thought was to completely eliminate the bacterial insult and the contaminated implant as the best strategy for the ongoing health of the entire maxillary restoration.
The implants were removed, the bone debrided, and the patient treated with systemic antibiotics for 10 days. Four months later, through a crestal incision, the vertical defect was found to range from 6 to 10 mm in height (Figure 4). A titanium mesh was fitted to framework height and width (Figure 5). The mesh was filled with mineralized allograft bone chips and BMP-2 impregnated collage in a 70:30 ratio. The graft-filled mesh was then positioned into place and fixed with titanium screws (Figure 6) and a resorbable collagen membrane placed over the crestal aspect. The wound was then closed in a watertight closure.
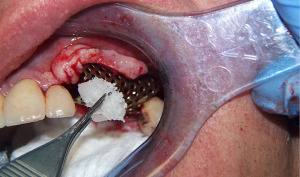
After 10 weeks, the mesh was found to be exposed. An X-ray showed mineralization of the graft site (Figure 7) so the mesh was removed (Figure 8) and the graft site was left to mature another 3 months for a total of 5 months since graft placement. At that time a crestal incision was made and two implants instead of three were placed. It was noted at the time of implant placement that bone levels had increased on the adjacent molar implant from the mesh graft but the defect site had lost 2 mm of bone in the area of mesh exposure. The overall height of bone gain was about 8 mm. Following the healing of the implants, the final restoration was completed (Figures 9,10). The new restoration length of crowns was slightly longer but alveolar width and gingival contours had been fully restored. (Figures 11,12) Five years later the reconstruction and peri-implant bone levels remained stable.
Case 2—failed implant-supported full-arch rehabilitation
A 55-year-old healthy female patient was presented with a full arch maxillary ceramo-metal bridge bonded to four dental implants. A fifth dental implant in the position of the upper left second incisor was removed several months prior to patient presentation due to severe bone loss around the implant (Figures 13-15).
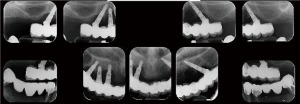
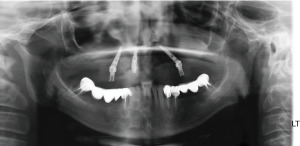
Upon examination, the patient suffered from generalized mucositis in the upper jaw soft tissue, accompanied with pain, spontaneous bleeding, and suppuration, with probing depth around all implants of up to 12 mm. The patient asked for a non-removable restoration, both during the treatment process and at its end.
The first phase of the treatment aimed to eradicate the peri-implant disease. Since the patient could not tolerate the use of removable dentures, the decision was to temporarily use three of the implants to support a temporary restoration. The implant surface areas were aggressively debrided, followed by air-powder abrasion and citric acid application. Three multi-units were adapted to the implants and an acrylic temporary bridge was screw fixated.
This course of treatment was intended to allow both soft and hard tissues to heal in order to prepare them for future bone augmentation and regeneration. In addition, the ability to temporarily keep the debrided and inflammation-free failing implants to support a fixed temporary restoration may assist in providing pressure and friction-free conditions for the future augmented areas.
After 3 months, the patient presented without inflammation, with an improved soft-tissue appearance. A bilateral sinus augmentation was performed using mineralized allograft bone chips and xenograft bone chips in a 50:50 ratio (Figure 16). Seven months later, three dental implants were installed on each side of the maxilla, and veneer augmentation of the frontal and peri-implant area was performed using allograft bone chips covered with a resorbable collagen membrane (Figure 17).
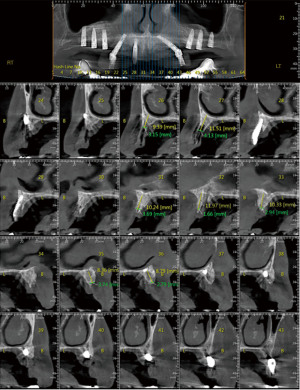
After 4 months, the three original implants were removed, and four new implants were installed in the frontal maxillary area. New multi-units were connected to the implants (except for the distal implants) for a new screw-retained temporary bridge (Figure 18).
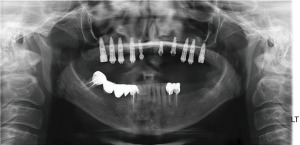
After 4 months, a final ceramo-metal screw-retained bridge was fixated (Figure 19).
A 4-year follow-up panoramic X-ray revealed good bone support for the implants (Figure 20).
The patient’s lower jaw was treated in a different practice.
Discussion
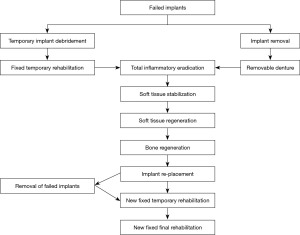
The key results of the two cases showing severe peri-implant disease necessitating removal of implants and inflamed bone suggests ablation and reconstruction. In other words, severe loss of bone from peri-implantitis is often so significant that the entire alveolus is lost, and retreatment becomes a significant challenge. Each such setting is by definition specific to the findings of the presenting lesion. The need for removal of implants prior to ablative injury is therefore implied by the two cases shown here as extensive reconstruction was required. Figure 21 suggests a workflow pattern for decision making on implant removal timing and subsequent reconstruction and reimplantation in severe peri-implant disease settings.
The decision to remove an implant with peri-implant disease is never easy. It may have a medical, functional, aesthetic, financial, and psychological impact, and should be made after a thorough consideration of the various implant treatment possibilities (21,22). The psychological effect of implant removal on patients may be hard for the patient and at times intolerable, especially when a fixed prosthesis must be replaced by a removable denture. Some patients will not be able to adjust to removable dentures, and this must be considered and discussed prior to any treatment plan (23).
There is not a standardized way to treat these patients in all the various situations possible. For example, an implant used for an overdenture support that has 50% bone loss may best be removed and replaced adjacently without bone grafting, this being the most conservative and least invasive approach. Whereas an implant supporting a fixed bridge with 50% bone loss with otherwise favorable and relatively healthy osseous support may be amenable for retention, even perhaps regrafting, due to its critical importance in the restorative scheme. After the removal of failed implants due to peri-implant disease, and especially when severe bone loss is identified, a comprehensive restorative re-treatment course should be planned. This is not similar to a treatment plan used for a similar edentulous area that is not contaminated with peri-implant disease. This is due to the specific characteristics and impact of the peri-implant disease on the surrounding soft and hard tissues, as well as on the predicted success rate of the re-placed implants (24).
In contrast to the popular approach in recent years of performing as many simultaneous surgical and prosthetic procedures as possible to allow for an expedited treatment course (25,26) , when re-treating a failed implant site with severe bone loss, a staged approach is advised. This should include a stepped course of inflammatory eradication, patient-specific factors treatment, soft and bony tissues regeneration and augmentation, replacement of implants, and prosthodontic rehabilitation (27).
Inflammatory eradication
The best way to eradicate peri-implantitis is to remove implants and debride the area in preparation for definitive retreatment. Sometimes implants can be debrided and regrafted but not when there is severe bone loss.
Peri-implantitis is defined as an inflammatory process due to bacterial plaque (28), most commonly Gram-negative anaerobes (Prevotella intermedia, Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, Tannerella forsythia, Treponema denticola, Prevotella nigrescens, Peptostreptococcus micros, and Fusobacterium nucleatum) (29,30). Removing the failed implants solely, without prompt attention to the contaminated surrounding tissues, will deem the re-treatment process to fail (31).
It is of crucial importance to thoroughly debride all inflammatory tissues at the site of implant failure. This should include aggressive bone tissue curettage, including removal of any infected bone substitute that may be present until viable and bleeding bone is evident at the periphery (32). Soft tissue should be handled gently. It is advisable not to remove soft tissues, even when infected, since the potential of soft tissue healing in a bacterial-free environment is high (33), and the availability of sufficient viable soft tissue is of great importance for the success of any regenerative treatment.
Treatment of patient-specific factors
Before re-treating failed implant cases, it is important to evaluate and treat patient-specific factors that may have caused or contributed to the implant failure or may affect the potential of the re-treatment success. Patients must undergo meticulous and frequent peri-implant and periodontal therapy as needed and commit to persist with this preventative regimen after re-treatment. Smoking patients should be given clear and sufficient data and explanation regarding the contributing effect of smoking on implant failure and advised to quit or reduce smoking (34).
In cases of bruxism, patients should be treated with load-reducing appliances to reduce the impact of over-loading to the peri-implant bone (35). Diabetic patients must be monitored and controlled, and high glucose levels should be treated prior to the implant re-treatment (36,37).
In cases of patients treated with antiresorptive medications for osteoporosis or other bone-related diseases, the re-treatment protocol must adhere to the 2014 American Association of Oral and Maxillofacial Surgeons (AAOMS) position paper on the prevention of medication-related osteonecrosis of the jaws (38).
In general, as mentioned above, without identifying and treating the patient-specific factors that may have been the source for the peri-implant disease, there may be a higher chance for the failure of the re-treatment process.
Soft and bony tissue regeneration
The limitation of this study is the limited data provided by two cases. However, there are not very many reports, and indeed that many cases, of these findings described here in any one institution where there is such extensive treatment required especially in the case of complete alveolar generation.
The key factors for successful bony regeneration include: (I) good blood supply (including a good supply of osteoprogenitor cells, growth factors, and bone morphogenic proteins) (39,40); (II) good stable envelope for three-dimensional guidance, and physical enclosure and protection over the regenerative bone materials (41). When there is not enough soft tissue to envelop and fully cover the regenerative bone materials, this should first be regenerated with various techniques offered for that purpose. This is important since the soft tissues give the main source for blood supply and cellular supply (42). In addition, watertight coverage over the regenerative bone materials protects them from contamination with bacteria from the oral cavity and possible failure due to infection (43). When there is a risk for the three-dimensional stability of the regenerative bone materials, a rigid envelope, such as a titanium mesh, should be adapted to protect the desired spatial form of the re-treated area (44). It is important to note, that after the first mineralization of the regenerated bone, the rigid envelope may be removed, especially if it challenges the durability of the soft tissue closure over the bone graft (45).
When large defects are re-treated, especially when allograft or xenograft materials are used, it may be advocated to add BMPs or growth factors to assist the bone regeneration process as was done here in the one case (46-49).
Re-placement of implants and prosthodontic rehabilitation
Due to the potential psychological difficulty of patients after implant removal, and in face of long-term re-treatment periods, special thought should be given to the temporary rehabilitation. When re-treating posterior segments, outside of the aesthetic zone, it is important to make sure that patient functions, like eating, speaking, and talking, are not compromised (50). When the re-treated segment includes the aesthetic zone, this must be also addressed with the temporary restoration (51).
In cases of failing implants, with preserved stability, de-contamination of part of the implants to temporarily support fixed temporary rehabilitation may be considered as was shown here in one case. Debridement may be performed using various surgical, mechanical, and medical techniques. This may psychologically assist patients to better adjust to the re-treatment. Also, this has the advantage to preclude the unfavorable loading from the removable dentures on the augmented re-treated site (52).
When re-placing implants in a re-treated area it is most important to keep a sufficient distance between implants, with as accurate prosthetic derived positioning as possible. This is to minimize any ischemic or overload potential to the regenerated bone (53,54). In addition, in cases of additional implant failure due to peri-implant disease, this may minimize the risk for implant-to-implant contamination due to proximity (24). The prosthetic rehabilitation of re-treated cases is similar to every other implant-supported rehabilitation.
The key conclusions from the study are staging treatment such that reimplantation is not done at the time of implant removal and that the osseous bed is prepared and left to heal for a period of time so that graft reconstruction can be done dependably. Retreatment implants then follow later, often several months later. The rational for this patient and cautious approach is decontamination of both hard and soft tissues as reimplantation success rate is always lower than initial treatment.
Further study needs to address the best timing for removal of implants so that alveolar ablation does not occur obviating the need for bone graft reconstruction. In addition, improved methods for treating failing implants and perhaps regenerating bone around peri-implant diseased implants should be investigated.
Summary
Peri-implant disease may result in loss of bone support and a need for implant removal. In certain cases, a significant bone volume may be lost leaving the patient in a state of alveolectomy, impossible for reimplantation without significant bone graft augmentation.
When re-treating such failed implant sites, a comprehensive approach should be applied. This must include a stepwise, controlled process of complete inflammatory eradication, attention to patient-specific factors that may contribute to implant failure, regeneration of soft and bony tissues, and implant re-placement followed by suitable fixed rehabilitation.
Special care must be given to the psychological effects of implant failure and removal and the long period that the re-treatment course may take, especially with regard to the possibility of using fixed temporary rehabilitation when feasible.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Frontiers of Oral and Maxillofacial Medicine, for the series “Current Advances in Treatment of Peri Implant Disease”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the PROCESS reporting checklist. Available at https://fomm.amegroups.com/article/view/10.21037/fomm-21-111/rc
Conflicts of Interest: All the authors have completed the ICMJE uniform disclosure form (available at https://fomm.amegroups.com/article/view/10.21037/fomm-21-111/coif). The series “Current Advances in Treatment of Peri Implant Disease” was commissioned by the editorial office without any funding or sponsorship. OTJ served as the unpaid Guest Editor of the series and serves as the unpaid editorial board member of Frontiers of Oral and Maxillofacial Medicine from December 2021 to November 2023. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The manuscript does not require an ethical approval. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Patient permission for publication of pictures and data was obtained. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Moraschini V, Poubel LA, Ferreira VF, et al. Evaluation of survival and success rates of dental implants reported in longitudinal studies with a follow-up period of at least 10 years: a systematic review. Int J Oral Maxillofac Surg 2015;44:377-88. [Crossref] [PubMed]
- Greenstein G, Cavallaro J, Romanos G, et al. Clinical recommendations for avoiding and managing surgical complications associated with implant dentistry: a review. J Periodontol 2008;79:1317-29. [Crossref] [PubMed]
- Berglundh T, Armitage G, Araujo MG, et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol 2018;89:S313-8. [Crossref] [PubMed]
- Muñoz V, Duque A, Giraldo A, et al. Prevalence of Peri-implant Disease According to Periodontal Probing Depth and Bleeding on Probing: A Systematic Review and Meta-Analysis. Int J Oral Maxillofac Implants 2018;33:e89-105. [Crossref] [PubMed]
- Renvert S, Lindahl C, Persson GR. Occurrence of cases with peri-implant mucositis or peri-implantitis in a 21-26 years follow-up study. J Clin Periodontol 2018;45:233-40. [Crossref] [PubMed]
- Derks J, Tomasi C. Peri-implant health and disease. A systematic review of current epidemiology. J Clin Periodontol 2015;42:S158-71. [Crossref] [PubMed]
- Derks J, Schaller D, Håkansson J, et al. Effectiveness of Implant Therapy Analyzed in a Swedish Population: Prevalence of Peri-implantitis. J Dent Res 2016;95:43-9. [Crossref] [PubMed]
- de Waal YC, van Winkelhoff AJ, Meijer HJ, et al. Differences in peri‐implant conditions between fully and partially edentulous subjects: a systematic review. J Clin Periodontol. 2013;40:266-86. [Crossref] [PubMed]
- Greenstein G, Cavallaro J. Failed dental implants: diagnosis, removal and survival of reimplantations. J Am Dent Assoc 2014;145:835-42. [Crossref] [PubMed]
- Misch CE, Perel ML, Wang HL, et al. Implant success, survival, and failure: the International Congress of Oral Implantologists (ICOI) Pisa Consensus Conference. Implant Dent 2008;17:5-15. [Crossref] [PubMed]
- Gomes GH, Misawa MYO, Fernandes C, et al. A systematic review and meta-analysis of the survival rate of implants placed in previously failed sites. Braz Oral Res 2018;32:e27. [Crossref] [PubMed]
- Meffert RM. Periodontitis vs. peri-implantitis: the same disease? The same treatment? Crit Rev Oral Biol Med 1996;7:278-91. [Crossref] [PubMed]
- Becker ST, Beck-Broichsitter BE, Graetz C, et al. Peri-implantitis versus periodontitis: functional differences indicated by transcriptome profiling. Clin Implant Dent Relat Res 2014;16:401-11. [Crossref] [PubMed]
- Monje A, Pons R, Insua A, et al. Morphology and severity of peri-implantitis bone defects. Clin Implant Dent Relat Res 2019;21:635-43. [Crossref] [PubMed]
- Rakic M, Lekovic V, Nikolic-Jakoba N, et al. Bone loss biomarkers associated with peri-implantitis. A cross-sectional study. Clin Oral Implants Res 2013;24:1110-6. [Crossref] [PubMed]
- Singh P. Understanding peri-implantitis: a strategic review. J Oral Implantol 2011;37:622-6. [Crossref] [PubMed]
- Galárraga-Vinueza ME, Tangl S, Bianchini M, et al. Histological characteristics of advanced peri-implantitis bone defects in humans. Int J Implant Dent 2020;6:12. [Crossref] [PubMed]
- Emecen-Huja P, Eubank TD, Shapiro V, et al. Peri-implant versus periodontal wound healing. J Clin Periodontol 2013;40:816-24. [Crossref] [PubMed]
- Chrcanovic BR, Kisch J, Albrektsson T, et al. Survival of dental implants placed in sites of previously failed implants. Clin Oral Implants Res 2017;28:1348-53. [Crossref] [PubMed]
- Oh SL, Shiau HJ, Reynolds MA. Survival of dental implants at sites after implant failure: A systematic review. J Prosthet Dent 2020;123:54-60. [Crossref] [PubMed]
- Tarnow DP, Chu SJ, Fletcher PD. Clinical Decisions: Determining When to Save or Remove an Ailing Implant. Compend Contin Educ Dent 2016;37:233-243;quiz244.
- Robertson K, Shahbazian T, MacLeod S. Treatment of peri-implantitis and the failing implant. Dent Clin North Am 2015;59:329-343. [Crossref] [PubMed]
- Kent G, Johns R. Psychological effects of permanently implanted false teeth: a 2-year follow-up and comparison with dentate patients. Psychology & Health 1993;8:213-22.
- Prathapachandran J, Suresh N. Management of peri-implantitis. Dent Res J (Isfahan) 2012;9:516-21. [Crossref] [PubMed]
- Covani U, Barone A, Cornelini R, et al. Clinical outcome of implants placed immediately after implant removal. J Periodontol 2006;77:722-7. [Crossref] [PubMed]
- Anitua E, Orive G. A new approach for atraumatic implant explantation and immediate implant installation. Oral Surg Oral Med Oral Pathol Oral Radiol 2012;113:e19-25. [Crossref] [PubMed]
- Wang WC, Lagoudis M, Yeh CW, et al. Management of peri-implantitis - A contemporary synopsis. Singapore Dent J 2017;38:8-16. [Crossref] [PubMed]
- Algraffee H, Borumandi F, Cascarini L. Peri-implantitis. Br J Oral Maxillofac Surg 2012;50:689-94. [Crossref] [PubMed]
- Lafaurie GI, Sabogal MA, Castillo DM, et al. Microbiome and Microbial Biofilm Profiles of Peri-Implantitis: A Systematic Review. J Periodontol 2017;88:1066-89. [Crossref] [PubMed]
- Pérez-Chaparro PJ, Duarte PM, Shibli JA, et al. The Current Weight of Evidence of the Microbiologic Profile Associated With Peri-Implantitis: A Systematic Review. J Periodontol 2016;87:1295-304. [Crossref] [PubMed]
- Mombelli A, Lang NP. The diagnosis and treatment of peri-implantitis. Periodontol 2000 1998;17:63-76. [Crossref] [PubMed]
- Esposito M, Grusovin MG, Worthington HV. Treatment of peri-implantitis: what interventions are effective? A Cochrane systematic review. Eur J Oral Implantol 2012;5:S21-41.
- Nguyen-Hieu T, Borghetti A, Aboudharam G. Peri-implantitis: from diagnosis to therapeutics. J Investig Clin Dent 2012;3:79-94. [Crossref] [PubMed]
- DeLuca S, Habsha E, Zarb GA. The effect of smoking on osseointegrated dental implants. Part I: implant survival. Int J Prosthodont 2006;19:491-8.
- Lobbezoo F, Brouwers JE, Cune MS, et al. Dental implants in patients with bruxing habits. J Oral Rehabil 2006;33:152-9. [Crossref] [PubMed]
- Katyayan PA, Katyayan M, Shah RJ. Rehabilitative considerations for dental implants in the diabetic patient. J Indian Prosthodont Soc 2013;13:175-83. [Crossref] [PubMed]
- Park JB. Bone healing at a failed implant site in a type II diabetic patient: clinical and histologic evaluations: a case report. J Oral Implantol 2007;33:28-32. [Crossref] [PubMed]
- Ruggiero SL, Dodson TB, Fantasia J, et al. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw--2014 update. J Oral Maxillofac Surg 2014;72:1938-56. Erratum in: J Oral Maxillofac Surg 2015;73:1440; J Oral Maxillofac Surg 2015;73:1879. [Crossref] [PubMed]
- Wang HL, Boyapati L. "PASS" principles for predictable bone regeneration. Implant Dent 2006;15:8-17. [Crossref] [PubMed]
- Mattout P, Mattout C. Conditions for success in guided bone regeneration: retrospective study on 376 implant sites. J Periodontol 2000;71:1904-9. [Crossref] [PubMed]
- Fugazzotto PA. Maintenance of soft tissue closure following guided bone regeneration: technical considerations and report of 723 cases. J Periodontol 1999;70:1085-97. [Crossref] [PubMed]
- Wang Y, Zhang Y, Miron RJ. Health, Maintenance, and Recovery of Soft Tissues around Implants. Clin Implant Dent Relat Res 2016;18:618-34. [Crossref] [PubMed]
- Liu J, Kerns DG. Mechanisms of guided bone regeneration: a review. Open Dent J 2014;8:56-65. [Crossref] [PubMed]
- Rakhmatia YD, Ayukawa Y, Furuhashi A, et al. Current barrier membranes: titanium mesh and other membranes for guided bone regeneration in dental applications. J Prosthodont Res 2013;57:3-14. [Crossref] [PubMed]
- Xie Y, Li S, Zhang T, et al. Titanium mesh for bone augmentation in oral implantology: current application and progress. Int J Oral Sci 2020;12:37. [Crossref] [PubMed]
- Fernandez de Grado G, Keller L, Idoux-Gillet Y, et al. Bone substitutes: a review of their characteristics, clinical use, and perspectives for large bone defects management. J Tissue Eng 2018;9:2041731418776819. [Crossref] [PubMed]
- Cook SD, Baffes GC, Wolfe MW, et al. The effect of recombinant human osteogenic protein-1 on healing of large segmental bone defects. J Bone Joint Surg Am 1994;76:827-38. [Crossref] [PubMed]
- Sharmin F, O'Sullivan M, Malinowski S, et al. Large scale segmental bone defect healing through the combined delivery of VEGF and BMP-2 from biofunctionalized cortical allografts. J Biomed Mater Res B Appl Biomater 2019;107:1002-10. [Crossref] [PubMed]
- Casap N, Rushinek H, Jensen OT. Vertical Alveolar Augmentation Using BMP-2/ACS/Allograft with Printed Titanium Shells to Establish an Early Vascular Scaffold. Oral Maxillofac Surg Clin North Am 2019;31:473-87. [Crossref] [PubMed]
- Kinsel RP, Liss M. Retrospective analysis of 56 edentulous dental arches restored with 344 single-stage implants using an immediate loading fixed provisional protocol: statistical predictors of implant failure. Int J Oral Maxillofac Implants 2007;22:823-30.
- Kinsel RP, Lamb RE, Moneim A. Development of gingival esthetics in the edentulous patient with immediately loaded, single-stage, implant-supported fixed prostheses: a clinical report. Int J Oral Maxillofac Implants 2000;15:711-21.
- Burns DR, Beck DA, Nelson SK, et al. A review of selected dental literature on contemporary provisional fixed prosthodontic treatment: report of the Committee on Research in Fixed Prosthodontics of the Academy of Fixed Prosthodontics. J Prosthet Dent 2003;90:474-97. [Crossref] [PubMed]
- Al Amri MD. Influence of interimplant distance on the crestal bone height around dental implants: A systematic review and meta-analysis. J Prosthet Dent 2016;115:278-82.e1. [Crossref] [PubMed]
- Elian N, Bloom M, Dard M, et al. Effect of interimplant distance (2 and 3 mm) on the height of interimplant bone crest: a histomorphometric evaluation. J Periodontol 2011;82:1749-56. [Crossref] [PubMed]
Cite this article as: Alterman M, Jensen OT, Mazor D, Casap N. Re-treatment of failed maxillary implant sites with severe bone loss from peri-implant disease: case series. Front Oral Maxillofac Med 2023;5:10.