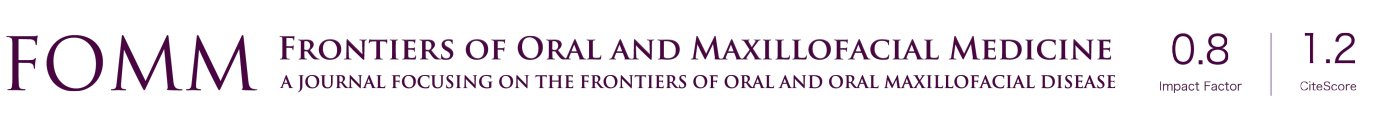Airway-centric orthodontics: a review on oral appliance therapy as a simplified solution to obstructive sleep apnea
Introduction
Background
“You that seek what life is in death, now find it air that once was breath.”—Baron Brooke Fulke Greville, “Caelica 83”.
Air, breath, and life are synonymous. Breath sustains life and every cell of the several billion that constitute the human body’s need to breathe. Surprising though, the intersection of evolutionary biology, function, and biomechanical modulation of the craniofacial skeleton struggles to find a central position in clinical orthodontics and oral health sciences.
Any decrease in breath on the other hand has numerous dangerous and potentially dangerous effects for all life. Obstructive sleep apnea (OSA) is one such disease characterized by episodes of upper air passage collapse during sleep accompanied by hypoxia and sleep arousals. Patients with OSA may complain of various signs and symptoms such as daytime sleepiness (1), snoring and choking and gasping during sleep (2), nocturia (3), morning headaches (4), increased sedentary fatigue (5), unrefreshing sleep (6), and chronic insomnia (7) making a positive diagnosis of OSA difficult (8). Risk factors predisposing to OSA include obesity, large neck diameter, male sex, increasing age, post-menopausal status, and disturbances of the craniofacial region (6,9).
While polysomnography (PSG) remains the gold standard for testing for OSA, the use of home sleep apnea testing (HSAT) has emerged as a reasonable alternative with a sensitivity and specificity of 79% the use of several questionnaires such as the Stop Bang, Epworth Sleepiness Scale (ESS), and the Berlin Scale also have seen a wide acceptance in the clinical setting (6).
Rationale and knowledge gap
OSA prevalence is increasing, Benjafield et al. reported an OSA (mild to severe) prevalence of 936 million persons between 30 to 69 years, and 425 million persons between 30 and 69 years have OSA (moderate to severe) globally, according to the diagnostic criteria of the American Academy of Sleep Medicine (10). The prevalence rates of OSA in different regions vary with rates from 3% (females) to 10% (males) (11) to rates as high as 78% (females) to 90% (males) being reported making it one of the commonest sleep disorders (12). The rising challenge of obesity brings an increasing focus on OSA. There is a need to address the address the etiology, pathogenesis, and management strategies for OSA. The role craniofacial anomalies have been receiving much attention as contributors for OSA (6), with a lot of recent studies focusing on the role of malocclusion and craniofacial anomalies as contributory factors for OSA bringing to fore the role of dentists in early diagnosis and management of OSA (13,14).
There exist diverse modalities for the management of OSA with lifestyle modifications such as abstinence from alcohol, exercise, changes in sleep position to name a few being the least invasive (6). Skeletal and soft tissue surgeries have also been recommended as management strategies (15). Positive air pressure (PAP) has emerged as the mainstay in the treatment of OSA. Continuous PAP (CPAP) was first tried in 1981 and is the most common delivery in use subsequent modifications such as bilevel PAP (BPAP) have provided better results. Technological advancements such as auto CPAP (ACPAP) and Auto BPAP (ABPAP) have led to better results (16). However patient compliance with PAP devices have been challenging. There is also the emerging recognition that effective management of OSA needs an interdisciplinary approach (17).
Dentists often examine various structures such as the tongue, tonsils, soft palate and evaluate jaw position routinely thus making them an important part of the multidisciplinary team for the management of OSA (18). The use of oral appliances which reposition the jaws during sleep in the management of OSA is a promising field because of better tolerance in patients intolerant to PAP appliances (6) leading to the emergence of a new field in dentistry i.e., dental sleep medicine.
Objective
This review presents an all-inclusive view of OSA in the context of evolutionary cranio-skeletal development, collates current knowledge regarding OSA prevalence in contrast to issue or subject specific reviews. The biologic effects of OSA have been added to ensure a holistic view of the condition. Special emphasis that has been placed on the recommendations on diagnosis of OSA using simple diagnostic aids routinely used by dentists and management of OSA using relatively simple oral appliance therapy (OAT) are deliberated.
OSA: cause & ramifications
The term “Pickwickian syndrome” that’s sometimes used for the syndrome became coined by the famous early twentieth-century doctor, William Osler, who apparently was a reader of Charles Dickens. The description of Joe, “the fat boy” in the pickwick papers suits flawlessly with the clinical image of a person with OSA syndrome. The effect of OSA on overall health is also well established, 50% of cardiac patients have a varying degree of OSA and a causative link between OSA, stroke, and coronary artery disease is also well established in literature (19,20). Yet the underlying problems remain surprisingly undiagnosed. A systematic review and meta-analysis by Chen et al. in 2021 shows that 80–90% of cases go undiagnosed with a huge health liability and risk (21). There is flippancy and embarrassment over seeking help or a consultation over sleep problems and snoring.
OSA is related to the enhanced crumpling of the upper air passage. The pharyngeal critical closing pressure (PCRIT) defines the point at which the air passage collapse occurs. Impaired muscle tone, mandibular and tongue position add to the collapse. The increased effort to maintain respiration in a compromized air passage is accompanied by an enhanced serum carbon dioxide and diminished oxygen levels (22,23). The respiratory distress is related to cortical arousal and an increase in sympathetic neural activity with spikes in blood pressure, heart rate and a predisposition towards cardiac arrythmias (8). Multiple sleep wake cycles complicate the issue with serious health consequences.
Search strategy
PubMed/Embase/Scopus/Web of Science databases were searched for studies that mentioned sleep apnea, obstructive sleep apnea, OSA in the title and mentioned oral appliance, myofunctional in the title or abstract. The following search strategy was put into the advanced research intelligence system (AriS) chart using python software (Figure 1). The articles were sub divided into clinical trials, comparative studies, and systematic reviews and meta-analyses. The following were the search terms used: (sleep apnea [Title] OR obstructive sleep apnea[Title] OR OSA[Title]) AND (appliance[Title/Abstract] OR oral appliance[Title/Abstract]) AND (systematic review[Title] OR meta-analysis[Title]).
OSA: an evolutionary basis
Evolutionary biology has been a serious contributor to this emerging silent epidemic as some have termed it (24). Bipedalism, an erect posture, a collapsible oropharynx, all led to a progressive set of factors where a minor variation could have unprecedented outcomes on the airway and breathing. Bipedalism of homo sapiens came at a price, the scaffolding had to support the weight of the cranium, brain posture became an issue and the larynx descended. Migration into the grasslands and the introduction of pursuit hunting led to an increased demand for oxygen in our ancestors which was provided by mouth breathing. The process of verbal communication and language affected tongue position and posture. If the tongue rested completely in the oral cavity, it would not impede the airway, but phonation and articulation would be a challenge, so the tongue came to lie in the oropharynx setting the stage for compromising airway with even small variations in position and posture (25).
A growing concern in evolutionary biology was the possibility of a progressive reduction of the size of the human jaws, this is made out by comparisons of medieval skulls with modern skulls in which the medieval skulls have shown little or no evidence of malocclusion, had ample space for the tongue, and showed a minimal prevalence of mandibular third molar impactions, all of these traits are found compromized in modern human skulls. Craniofacial development was linked to changing dietary and chewing habits from coarse to more refined food (26) and anthropologists set about building a link with the reduction in the size of the jaws (27). Some experts in dental anthropology, infer that malocclusion has accelerated in the last 150 years or so in technologically advanced communities (28).
Observations on the effect on muscle posture modifying facial growth are present in published literature (29). Obstructions in the naso-respiratory passages have been found to promote a clockwise rotation in the growing mandible thereby leading to increase in anterior vertical facial height and decrease in posterior vertical height leading to retrognathia and open bites (30,31). Harvold has demonstrated the impact of impaired nasal breathing on craniofacial development. Obstructed airway leads to an extended cranio-cervical extension and a forward head posture to maintain patency of airway which unfortunately does not happen during sleep (32).
Dentists on the other hand have seen malocclusions from the prism of genetics only, ignoring an increasing body of evidence which focusses on the epigenetic/phenotypic events leading to malocclusions (33).
What Mew postulated in his philosophical paper suggesting the disruption of resting oral posture by environmental factors guiding skeletal growth patterns and genesis of a dental malocclusion which is defined by muscle patterns primarily of the tongue (34) has now found experimental evidence in the work of Engelke et al. who have demonstrated the formation at least two functional components in their bio-functional model reaffirming the role of muscle posture in oral skeletal development (35). These findings present an interesting evolutionary pathway for the development of OSA (Figure 2) and are summarized in Table 1.
Table 1
| Author, year | Study type | Number of subjects | Outcome evaluated | Results and conclusion | Quality of evidence |
|---|---|---|---|---|---|
| Kahn, 2020 | Expert opinion and literature review | – | – | OSA symptoms are phenotypic responses to a vast natural experiment—rapid and dramatic modifications of human physical and cultural environments | Low |
| Goose, 1962 | Case control | 403 | Width and length of modern and historic skulls | Significant reduction in width of modern skulls leading to crowding | Low |
| Corruccini, 1984 | Case control | 2,152 | Occlusal variations in overjet, overbite, crossbite and tooth displacement scores between urbanized and non-urban populations | Increased variations in overbite, crossbite and tooth displacement scores suggesting occlusal variations is an aberrancy of modern urbanized population | Low |
| Bresloin, 1983 | Case control | 45 | Effects of abnormal breathing patterns on facial growth using cephalometric radiographic analysis | Increased facial height, larger gonial angles, retrognathic maxilla and mandible, greater overjet and reduced maxillary intermolar width leading to increase molar crossbite seen in mouth breathers | Moderate |
| Nasal airway obstruction is associated with aberrant facial growth | |||||
| Harari, 2010 | Retrospective case control | 116 | Cephalometric analysis, and dental study models analysis for, arch form, tooth position, occlusion, and other normal parameters liable to undergo changes due to mouth breathing | The prevalence of a posterior cross bite was significantly more frequent in the mouth breathers group (49%) than nose breathers (26%) | Moderate |
| Abnormal lip-to-tongue anterior oral seal was significantly more frequent in the mouth breathers group (56%) than in the nose breathers group (30%) | |||||
| Naso-respiratory obstruction with mouth breathing during critical growth periods in children has a higher tendency for clockwise rotation of the growing mandible, with a disproportionate increase in anterior lower vertical face height and decreased posterior facial height | |||||
| D’Onofrio, 2019 | Expert opinion and literature review | – | Effect of oral dysfunction on malocclusion | Malocclusions and their acquired craniofacial dysmorphology are the result of chronic oral dysfunction and OMD | Low |
| Harvold, 1981 | Single center prospective | 42 | Relationship between mouth breathing and dental malocclusion by means of primate experiments | Increased facial height, larger gonial angle and steeper mandibular plane in mouth breathers | Moderate |
| Engelke, 2011 | Single center prospective | 20 | Effect of oral posture on the development of malocclusion by atmospheric pressure monitoring using a digital manometer in the vestibular interocclusal space and palatal vault | Demonstration of malocclusion related intraoral pressure levels as a basis for malocclusion therapy | Low |
OSA, obstructive sleep apnea; OMD, oral myofunctional disorder.
Biologic burden of OSA
The complex interrelationship between sleep and health has been widely recognized. Disturbed sleep is in numerous studies found associated with diseases of cardiovascular and metabolic systems. There is also emerging evidence that sleep disorders increase the risk of breast, prostate and colorectal cancers (36-40).
OSA has also been found to be associated with several systemic diseases and the intermittent hypoxia caused by OSA has been consistently found to predispose patients to cardiovascular diseases, insulin resistance and neural damage (8).
Cardiovascular diseases
OSA contributes to cardiovascular disease by affecting various paths of the cardiovascular system. Episodes of OSA produce central hemodynamic effects such as hypercapnia, intrathoracic pressure oscillation and produce arterial oxygen desaturation in addition to causing sleep disruption. This causes a fall in cardiac flow rate, decreased stroke volume and diminutions in cardiac rate (41). The post apnea period is typified by a pronounced transitory increase in systemic arterial pressure. The peripheral circulation is also affected with vasoconstriction being observed in the forearm and finger (42).
The increased cerebral blood flow seen during periods of apnea is followed by a sudden decline in the post-apnea period leading to fluctuations in the partial pressure carbon dioxide (PaCO2) and causing breathing disability during sleep (43). In the pulmonary circulation OSA produces cyclic patterns of vasoconstrictions and vasorelaxations leading to fluctuations in pulmonary arterial pressure (44).
Endothelial damage by induction of endothelial cell apoptosis (45), increased levels of cell death markers serum nucleosomes, double-stranded DNA (19) and the presence of increased levels of cellular adhesion molecules [intercellular adhesion molecule (ICAM), vascular cell adhesion molecule (VCAM), and E-selectin] all point to endothelial insult as a key event predisposing to the occurrence of other vascular events such as cardiovascular death, unstable angina and myocardial infarction with stratified hazard ratios as high as 6.01 for cardiovascular events occurring in OSA patients (46).
Insulin resistance
Knowledge that obesity is a factor in both OSA and insulin resistance leading to type 2 diabetes has led to postulations of relationships between OSA and insulin resistance (47), causal relationships between the two were however difficult to generate because of the presence of confounding factors such as smoking, body mass index (BMI), age, etc.
The nurses’ health study cohort found a relative risk for diabetes of 1.48 between occasional snoring vs. non-snoring, adjusting for other diabetes risk factors attenuated to relative risk to 1.41 showing that snoring and by extension OSA being an independent risk factor for type II diabetes (48). while the complex interrelationship between OSA and type II diabetes gets slowly unraveled the results of various clinical trials point out to intermittent hypoxia as a factor in causing insulin resistance and glucose intolerance in OSA patients.
Increased levels of catecholamines, the increased sympathetic neural traffic and amplified levels of cortisol by the effects of OSA on hypothalamic-pituitary-adrenal axis are other possibilities by which OSA predisposes to insulin resistance (49).
Neural damage
Neurobehavioral impairments such as sleepiness, fatiguability, impaired memory and poor concentration are seen in most adults with OSA. While investigating reasons for these, Macey et al. (50), Morrell et al. (51) have found marked reductions in the gray matter of the hippocampus, cingulate cortex and Broca’s area using magnetic resonance imaging (MRI).
Higher levels of brain infarctions (25%) in comparison to normal obese controls (6%) and increased levels of CD40 ligand (CD40L) and P-selectin markers for cerebrovascular disease have been demonstrated by Minoguchi et al. in persons with OSA (52).
In the peripheral nervous system consistent functional impairment, with demyelination of motoneurons has been found in OSA and a correlation between the oxyhemoglobin desaturation and the severity of peripheral nerve dysfunction have been observed (8).
OSA and the immune system
The sleep immune crosstalk has recently received much attention (53) and thus it would only be logical that OSA would lead to impairments in the immune system.
Increase in T helper cells and alteration in the Thelper/Tregulator ratios are noted in patients with OSA. Significant reductions in the peripheral dendritic cell population and reductions in B lymphocytes have also been found in patients suffering from OSA pointing to the presence of immune impairment in OSA (54).
In addition to impairments in the immune system OSA has also been shown to cause a dysregulation of circulating cytokines. Pro-inflammatory cytokines such as the interleukins 6, 12, 17, and 23, tumor necrosis factor alpha, and matrix metalloproteinase (MMP)3 were increased whereas MMP2 and tumor necrosis factor-like weak inducer of apoptosis (TWEAK) levels were decreased (55).
While the data on immune dysregulation in OSA patients is rather recent, immune dysregulation also seems to predispose OSA patients to certain types of cancers and influence oncological outcome (56,57).
The results of the quoted literature are consolidated in Table 2.
Table 2
| Author, year | Study type | Number of subjects | Outcome evaluated | Results and conclusion | Quality of evidence |
|---|---|---|---|---|---|
| Li, 2019 | Single center prospective multicentric | 622 | Effect of OSA on DNA methylation-based marker of biological aging | Severe OSA was associated with epigenetic age accelerators with AHI and arousal index associated with greater aging. Effects are more prominent among males | Moderate |
| OSA and cancer | |||||
| Cao, 2022 | Literature review and meta-analysis | 22 articles | Relationship between OSA and cancer | Overall prevalence of OSA among cancer patients is 46 and occurrence of cancers among persons suffering from OSA is 1.53 times higher than non-OSA individuals | Moderate |
| Blask, 2009 | Literature review | – | Lack of sleep and cancer risk | Shortened duration of nocturnal sleep associated with increased cancer risk | Low |
| Wong, 2021 | Prospective multicentric study and meta-analysis | 24,476 | Sleep duration and breast cancer risk over a period of 14.3 years | No correlation between sleep duration and risk of breast cancer | High |
| Almendros, 2012 | Single center prospective | 15 | Effect of high-rate intermittent hypoxia seen in OSA on tumor size in a murine melanoma model | Two-fold increase in tumor necrosis a size in the hypoxic rats | Low |
| Intermittent hypoxia enhances tumor growth | |||||
| Huppertz, 2021 | Single center prospective | 33 | HSAT in patients with confirmed oropharyngeal carcinomas and comparison with primary tumor size | Significant correlation between primary tumor size and AHI | Low |
| Nieto, 2012 | Multicenter retrospective cohort | 1,522 | Mortality data from Wisconsin sleep cohort to determine effect of OSA in cancer mortality | Adjusted relative hazards of cancer mortality: 1.1 for mild OSA, 2.0 for moderate OSA, 4.8 for severe OSA | Moderate |
| OSA is associated with increased cancer mortality | |||||
| Choi, 2014 | Single center retrospective | 45,699 | Associations between OSA and breast cancer incidence using the Korea national health insurance service database | Increased risk of breast cancer in OSA patients with a HR 1.20 (1.04–1.29) and increasing to 1.72 (1.10–2.19) in patients >65 years of age | – |
| OSA and the CVS | |||||
| Shiomi, 1991 | Single center prospective | 10 | Echocardiogram from parasternal long axis view in OSA patients | Left shift of the intraventricular septum with pulsus paradoxus which disappeared after CPAP treatment | Low |
| Leuenberger, 2001 | Single center prospective | 10 | Heart rate, MAP, MSNA (peroneal microneurography), and VFA (Doppler) under hypoxia (10.5% inspiratory fraction of O2), and hyperoxia (100% inspiratory fraction of O2) | The transient apnea-induced surges of MSNA and arterial pressure are associated with transient peripheral vasoconstriction | Low |
| Hypoxia accentuates the sympathetic and vasoconstrictor responses to apnea | |||||
| Xie, 2006 | Single center prospective | 9 | Oral indomethacin was used to reduce the cerebrovascular reactivity to CO2 and the steady-state hypercapnic ventilatory response to CO2 was tested and hyperoxia (50% O2) | Reduced cerebrovascular reactivity will cause an increase in ventilatory responsiveness to CO2, which is commonly seen during sleep in patients with central sleep apnea | Low |
| Weitzenblum, 2005 | Literature review | – | Relation between pulmonary hypertension and OSA | Nocturnal hypoxia not sufficient to induce pulmonary hypertension | Low |
| Solh, 2007 | Single center case control | 24 | Brachial artery flow-mediated dilation was determined in 14 subjects with documented OSA and 10 healthy control subjects at baseline and 8 weeks after CPAP therapy. Quantification of circulating apoptotic endothelial cells (CD146 Annexin V) was performed by flow cytometry | In patients with OSA, impairment of endothelial-dependent vasodilation correlated with the degree of endothelial cell apoptosis | Low |
| CPAP therapy led to significant decline in circulating apoptotic endothelial cells | |||||
| Peres, 2021 | Single center cohort | 418 | Predictive value of ICAM-1, VCAM-1, E-selectin levels in identifying risk of cardiovascular events in OSA | Higher levels of ICAM-1 were associated with developing CVD (HR =3.65, 95% CI: 1.40–9.53, 2nd and 3rd tertiles vs. 1st tertile), including in patients with OSA (HR =3.1, 95% CI: 1.16–8.25) | Moderate |
| E-selectin was significantly associated with cardiovascular events in patients with moderate to severe OSA (HR =3.31, 95% CI: 0.94–1.00, 2nd and 3rd tertiles vs. 1st tertile) but not in patients without moderate to severe OSA (HR =0.67, 95% CI: 0.19–2.38), P value for interaction =0.07 | |||||
| OSA and diabetes | |||||
| Al-Delaimy, 2002 | Prospective cohort | 1,957 | Association between snoring and type II diabetes | In analyses adjusted for age and BMI, snoring was associated with risk of diabetes [for occasional snoring vs. non-snoring, RR =1.48 (95% CI: 1.29, 1.70); for regular snoring vs. non-snoring, RR =2.25 (95% CI: 1.91, 2.66); P for trend <0.0001] | Moderate |
| Further adjustment for other diabetes risk factors and sleeping-related covariates only slightly attenuated the risk [for occasional snoring, RR =1.41 (95% CI: 1.22, 1.63); for regular snoring, RR =2.03 (95% CI: 1.71, 2.40); P for trend <0.0001] | |||||
| Punjabi, 2004 | Milticenter cohort | 2,656 | Comparisons of PSG and oral glucose tolerance tests | In comparison to controls subjects with mild SDB (5.0–14.9 events/h) and moderate to severe SDB (≥15 events/h) had adjusted odds ratios of 1.27 (95% CI: 0.98, 1.64) and 1.46 (95% CI: 1.09, 1.97), respectively, for fasting glucose intolerance (P for trend <0.01) | Moderate |
| Sleep-related hypoxemia was also associated with glucose intolerance independently of age, gender, BMI, and waist circumference | |||||
| The results of this study suggest that SDB is independently associated with glucose intolerance and insulin resistance and may lead to type 2 diabetes mellitus | |||||
| Brain changes in OSA | |||||
| Macey, 2002 | Single center case control | 42 | Comparison of high-resolution T1-weighted MRI between 21 OSA patients and 21 controls | Diminished regional and often unilateral gray matter in multiple sites of the brain in patients with OSA | Low |
| Unilateral loss in well-perfused structures suggests onset of neural deficits early in the OSA syndrome | |||||
| The gray matter loss occurs within sites involved in motor regulation of the upper airway as well as in areas contributing to cognitive function | |||||
| Morrell, 2003 | Single center case control | 14 | Voxel based morphometry comparison of structural changes in gray between patients diagnosed with OSA and non-OSA controls | Significantly lower gray matter concentration within the left hippocampus (P=0.004) in the apnoeic patients | Low |
| Minoguchi, 2007 | Single center case control | 89 | Silent brain infarction in 50 cases and 15 controls compared by MRI with 3-month CPAP treated follow up in 24 OSA cases. Levels of sCD40L and sP-selectin | The percentage of silent brain infarction in patients with moderate to severe OSA (25.0%) was higher than that of obese control subjects (6.7%) or patients with mild OSA (7.7%) | Low |
| Higher levels of serum markers in patients with moderate to severe OSA than in obese control subjects | |||||
| Reduction of serum marker levels with CPAP therapy | |||||
| Effects of OSA on the immune system | |||||
| Besedovsky, 2019 | Literature review | Relationship between sleep and immunity | Sleep affects innate and adaptive immunity | Low | |
| Ludwig, 2022 | Literature review | Relationship between OSA and cell mediated immunity | OSA has a significant impact on the cellular immune system | Low | |
| OSA and markers of renal disease | |||||
| Wang, 2021 | Single center prospective | 46 | Serum levels of MMP2, TWEAK, IL-6, and TNF-α (predictive markers of renal and cardiovascular diseases) using a BioPlex array in 19 cases of untreated OSA and 19 treated cases and 8 non-OSA controls | Treated OSA patients had a 5.4-fold higher median level of MMP2 (P=9.1×10−11), 1.4-fold higher level of TWEAK (P=1.8×10−7), 1.7-fold higher level of CD163 (P=1.4×10−6), in contrast to untreated patients | Low |
| Treatment brought about a significant normalization in levels | |||||
OSA, obstructive sleep apnea; AHI, apnea-hypopnea index; HSAT, home sleep apnea testing; CVS, cardiovascular system; CPAP, continuous positive air pressure; MAP, mean arterial pressure; MSNA, muscle sympathetic nerve activity; VFA, femoral artery blood velocity; ICAM, intercellular adhesion molecule; VCAM, vascular cell adhesion molecule; CVD, cardiovascular disease; HR, hazard ratio; CI, confidence interval; BMI, body mass index; RR, relative risk; PSG, polysomnography; SDB, sleep disordered breathing; MRI, magnetic resonance imaging; sCD40L, soluble CD40 ligand; sP, soluble P; MMP2, matrix metalloproteinase 2; TWEAK, tumor necrosis factor-like weak inducer of apoptosis; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α.
Malocclusion and its relation to OSA
Recent systematic reviews and metanalysis have found that the worldwide prevalence of malocclusion is 56% with no gender variances. Africa (81%) had the highest prevalence was seen in followed by Europe (72%), America (53%) lowest prevalence was seen in Asia (48%) with prevalence rates of class I (74.7%; range, 40–96%), class II (19.56%; range, 2–63%), and class III (5.93%; range, 1–20%) (58,59).
Craniofacial disharmony is reflected in cases of OSA. The most common features of disharmony seen in cases of OSA are bimaxillary retrusion, decreased mandibular length, reduced cranial base angle with a shortened cranial base and increased lower anterior facial height amongst these the decreased mandibular length has been found to dispose most to OSA (60,61). Multiple regression analysis have demonstrated increased overjet and a class II malocclusion as significant risk factors for OSA, however racial variations exist and lateral cross bite was the most prominent transverse dimension predisposing to OSA (13,61-63).
An elongated face with a dolichoprosopic pattern, steep mandibular plane angle, high arched narrow palate, also find a positive association with OSA (Figure 3) (64).
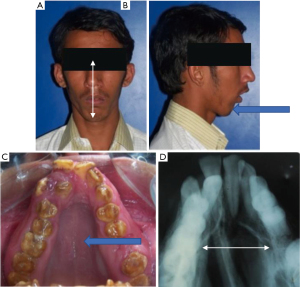
Considering all of these findings together, the classical pathogenesis begins to emerge. A narrow palate with a mouth breathing habit would imply increase nasal airway resistance and altered tongue posture. Increased overjet with crowding would prevent lip closure further aggravating the problem (65). The mandibular retrognathia associated with slackening of the genioglossus and mentalis muscles, hypotonia of the circumferent musculature would produce a fall back of the base of tongue in supine position with partial or complete airway obstruction (66). A skeletal class II pattern in patients with OSA would be associated with an underdeveloped/posteriorly positioned mandible with associated soft tissue and muscle attachments. The articles of this section are summarized in Table 3.
Table 3
| Author, year | Study type | Number of subjects | Outcome evaluated | Results and conclusion | Quality of evidence |
|---|---|---|---|---|---|
| Lombardo, 2020 | Systematic review and meta-analysis | 77 studies | Worldwide prevalence of malocclusion | Prevalence of malocclusion is 56% | High |
| The highest prevalence was in Africa (81%) and Europe (72%), followed by America (53%) and Asia (48%) | |||||
| Alhammadi, 2018 | Systematic review | 53 studies | Geographic and racial distribution of malocclusion and comparison between classes of malocclusion | The highest prevalence of class I was in Africa (89%), class II in Caucasian (23%) and class III in mongoloids | Moderate |
| Miles, 1996 | Literature review and meta-analysis | 143 studies | Etiological relevance of craniofacial structure to OSA | Only mandibular length, mandibular plane angle, and mandible to hyoid strongly correlated with OSA | Low |
| Banabilh, 2010 | Observational | 120 | Comparison of facial profile shape, malocclusion and palatal morphology in Malay adults with and without OSA as determined by AHI | Convex profile (71%) and class II malocclusion (51%) was more common in the OSA group | Low |
| Lam, 2005 | Observational case control | 239 | Whether craniofacial profile predicts presence of OSA as determined by AHI | Patients with OSA had higher thyromental angles and Mallampati scores than controls | Low |
| Johal, 2004 | Observational case control | 94 | Comparison of maxillary morphology between OSA and non-OSA patients | The palatal angle was more obtuse in male OSA subjects | Low |
| Minimum palatal airway widths were significantly reduced | |||||
| In the comparison of study model measurements, palatal heights in OSA subjects were greater | |||||
| Maxillary morphological differences do exist between OSA and control subjects, supporting their role as an etiological factor | |||||
| Jamieson, 1987 | Single center case control | 196 | Comparison of cephalometric radiographs and PSG of 155 cases and 41 controls to determine contribution of craniomandibular abnormalities to OSA | Retruded mandible, changed cranial base flexion and inferior hyoid bone displacement were commonly seen in OSA patients | Low |
OSA, obstructive sleep apnea; AHI, apnea-hypopnea index; PSG, polysomnography.
Defining airway centric orthodontics
Is health merely an absence of disease or infirmity? Could malocclusion fall into a definition of a disease? Orthodontics has struggled with its own identity of coming to terms with malocclusion and health (67). However, the ‘tooth’ paradigm has been centric to dentistry, the central focus being fixing crooked or diseased teeth and restoring an occlusion from a skill based and technique centric model using technology and materials. The industry has offered cookbook solutions with newer materials and a ’stepwise’ methodology often dictating professional trends. In the 1940’s the World Health Organization (WHO) proposed a larger definition of health correlating not just the absence of disease and infirmity but well-being and quality of life (68). Airway centric orthodontics brings into sharp focus the need for a link between orthodontics, oral medicine, sleep medicine, a concept of health with focus on cell anatomy, biology, physiology pathology and shifting a paradigm away from cosmetic or smile design which by association tend to diminish a specialty that is so deeply rooted in health and well-being. It brings a new paradigm of diagnosis and holistic treatment with appliances, techniques and technology being relegated to a secondary role (69). After all moving teeth and remodelling the craniofacial skeleton is a biologic and cellular phenomenon which could only work if all the cellular blocks are in harmony and alignment.
Diagnosis of OSA
OSA and sleep disordered breathing requires a joint workup with a physician to confirm the level of the problem. There has been a lot of debate over whether only sleep experts should be diagnosing and managing OSA, nevertheless OSA has been diagnosed and managed by confluence of specialties in dentistry and several studies have shown that the treatment outcomes do not vary on the type of expert diagnosing and managing OSA (70,71).
There is no physical examination that is specific for OSA, however the signs and symptoms (Table 4) that point out to the probability that a patient has OSA are as follows:
- Overweight/obese (72);
- Neck circumference (>17 male, >16 female) (72);
- Greater waist to height ratio (73);
- Male (6);
- Crowded oropharyngeal airway (74);
- Elder age group (6);
- Post menopausal status (6).
Table 4
| Symptom | Prevalence of symptom (%) |
|---|---|
| Daytime sleepiness | 73–90 |
| Snoring (loud and irregular) | 50–60 |
| Choking or gasping during sleep | 10–15 |
| Nocturia | 30 |
| Morning headaches | 10–30 |
| Insomnia | 29 |
| Lack of concentration/fatigue | 18–23 |
OSA, obstructive sleep apnea.
There exist several tools which would aid in assessment of OSA in a non-sleep clinical setting most sensitive among these is the Stop Bang questionnaire, essentially this looks at snoring (S), tiredness (T), observed pauses in breathing (O), blood pressure (P), the ESS (75) and the Berlin questionnaire are also used to predict OSA, however these alone should not be used to diagnose OSA (76,77).
Volumetric estimation of airway is possible by use of computed tomography (CT), MRI, acoustic imaging techniques and acoustic pharyngometry are in use today to estimate the degree and severity of OSA (78).
Home sleep testing/portable sleep testing reduce the necessity for patients to be tested in a sleep lab/hospital settings and recent introduction of devices such as Alice PDX, WatchPAT, etc. have provided results similar to lab PSG and are acceptable as alternatives to PSG in patients without comorbidities, home sleep testing devices report a respiratory event index (REI) in contrast to the PSG (79-82).
PSG however still remains the gold standard for identifying OSA and determining its severity (76,83). PSG provides an apnea-hypopnea index (AHI) (84). Based on AHI scores, OSA can be grouped into four categories:
- Normal (AHI <5 events/h);
- Mild OSA (AHI 5–15 events/h);
- Moderate OSA (AHI 16–30 events/h);
- Severe OSA (AHI >30 events/h).
Mild to moderate OSA is amenable to OAT, severe and central apnea’s need CPAP, nasal CPAP or oscillating positive airway pressure (OPAP) support apart from a detailed medical investigation (85,86).
Though dentists gather a vast array of records including a detailed personal and family history and perform cephalometric and model analysis, these have been with the defined purpose of estimating space to bring about an optimal occlusion with a consequent smile and/or an aesthetic facial outcome. While the utility of various cephalometric and model analysis in the diagnosis of OSA has been recognized quite early (87,88), the entire focus on cephalometric and model analysis in routine practice has been on creating metrics for tooth movement and adjusting teeth in a given occlusion (69). The assessment of skeletal disharmony is routine but so is the tendency for looking at a camouflage and compromise even before offering a more aggressive surgical correction of the jaw bases. This tendency has found favor and is promoted by the industry who tend to define extraction decisions related to a particular appliance. There is an inherent need to bring airway centric focus in the process of diagnosis.
Dental treatment goals need a redefinition beyond teeth and smiles. Improvement of upper airway patency and promotion of nasal breathing should be a primary objective (85). It is possible to outline a simple airway inclusive process in dental evaluation as proposed by Jayan and Kadu (89).
- Preliminary screening in all patients, adults or children, using an ESS or similar Stop Bang analysis;
- Distinctive clinical indicators for a compromized airway;
- Lip incompetency with oral breathing;
- Crumpling of chin;
- Adenotonsillitis;
- Airway grading of Mallampati more than grade III;
- Dark circles under the eye;
- Distended uvula;
- Severe clockwise rotation of the mandible;
- Tiny nares and high and narrow nose;
- Macroglossia and crenations.
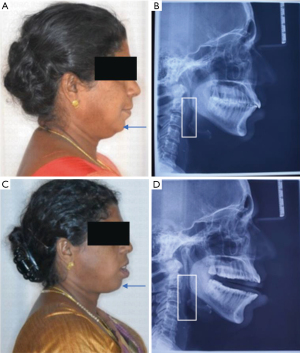
It is also imperative for dentists to leverage the routine records that are collated for diagnostics and treatment planning. A lateral cephalogram in natural head position provides good information with a number of analysis being available for assessing risk and potential airway collapsibility along with mandibular position, tongue position (Figure 4) (90). The inherent disadvantage is that the cephalogram is recorded in an erect posture and is two-dimensional (2D). Cone beam CT (CBCT) evaluations offer three-dimensional (3D) airway assessments apart from a physical estimation of the skeletal structures (91). The results of various studies quoted in this section are summarized in Table 5.
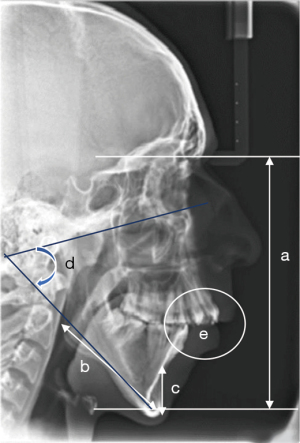
Table 5
| Author, year | Study type | Number of subjects | Outcome evaluated | Results and conclusion | Quality of evidence |
|---|---|---|---|---|---|
| Ken, 2018 | Systematic review | 9 articles | To assess OSA case-finding accuracy and comparative effectiveness of care by non–sleep specialists and sleep specialists | Similar outcomes of care by non–sleep specialists and sleep specialists. | Low |
| Tarraubella, 2018 | Multicenter RCT | 302 | Assessment of effectiveness and cost-effectiveness of primary care and sleep unit models for the management of subjects with suspected OSA | Similar outcomes in primary care and sleep unit models | Moderate |
| Epstein, 2009 | Expert opinion and clinical practice guidelines | – | Guidelines for the evaluation, management and long-term care of adult patients with OSA | Questions regarding OSA should be incorporated into routine health evaluations | High |
| Suspicion of OSA should trigger a comprehensive sleep evaluation | |||||
| The diagnostic strategy includes a sleep-oriented history and physical examination, PSG and education of the patient | |||||
| Unal, 2019 | Retrospective case control | 437 | Association between OSA syndrome and waist-to-height ratio | Cutoff values for OSAs were 95.5 cm for waist circumference, 0.595 for waist-to-height ratio Higher waist circumference and waist to height ratio associated with presence and severity of OSA |
Low |
| Ito, 2016 | Single center prospective | 703 | Correlations between cephalometric readings of tongue size, lower face cage and tongue size/lower face cage ratio with AHI | Tongue size/lower face cage ratio correlated with AHI | Low |
| Kapur, 2017 | Metanalysis and evidence-based task force clinical practice guideline | – | Systematic review was conducted to identify studies, and the GRADE process was used to assess the evidence. The task force developed recommendations and assigned strengths based on the quality of evidence. In addition, the task force adopted foundational recommendations from prior guidelines as “good practice statements”, that establish the basis for appropriate and effective diagnosis of OSA | The use of other clinical tools in the absence of PSG for OSA diagnosis contraindicated (strong) | High |
| PSG or HSAT is recommended for diagnosis moderate to severe OSA (strong) | |||||
| If HSAT results are negative or inconclusive a repeat PSG is recommended (strong) | |||||
| Chiu, 2017 | Bi variate meta-analysis | 100 studies | Comparison of the summary sensitivity, specificity, and diagnostic odds ratio among the Berlin, Stop Bang, Stop, and ESS according to the severity of OSA | The Stop Bang questionnaire is a better tool for detecting OSA when compared to other questionnaires | Moderate |
| Kendzerska, 2016 | Single center cohort | 576 | Comparison of UA-XSA and other clinical predictors of OSA using regression analysis | Mean UA-XSA was a predictor for OSA with an odds ratio of 1.62, but a regression analysis did not demonstrate and differences with other clinical predictors | Moderate |
| Ghegan, 2006 | Meta-analysis | 27 studies | Comparison of diagnostic of HAST vs. laboratory PSG | HSAT gave a 10% less RDI values odds ratio 0.90 | Moderate |
| HAST while providing similar diagnostic information underestimate OSA severity | |||||
| Gao, 2021 | Systematic review and meta-analysis using QUADAS-2 rating | 20 | Comparison of portable monitors and PSG in OSA diagnosis | Portable monitors showed a sensitivity of 74% and specificity of 90% when compared to PSG determined AHI | Low |
| Portable monitors provide an effective alternative to PSG | |||||
| Withers, 2022 | Single center prospective | 81 | Comparison of HSAT type 2 portable monitoring devices with type 1 PSG | Excellent correlation between the number of arousals, RDI, and sleep in both devices | Low |
| Type 2 devices are excellent for HSAT | |||||
| Cagle, 2023 | Meta-analysis | 24 studies | Comparison of AHI, ODI, RDI, O2 sat, and LSAT measured by PSSDs compared to PSG | Significant correlations between two devices of AHI, O2 sat, and LSAT | Moderate |
| Significant predictive correlation for AHI, RDI, and LSAT but not for O2 sat | |||||
| Borowiecki, 1988 | Observational case control | 42 | Comparison of cephalometric findings between patients with OSA and non-OSA controls | Patients with OSA had the following findings on cephalometric analysis: (I) enlarged tongue and soft palate; (II) the hyoid bone is displaced interiorly; (III) the mandible is normal in size and position (no micrognathia or malocclusion), and an elongated face caused by an inferior displacement of the mandibular body; (IV) retropositioned maxilla and elongated hard palate; (V) the nasopharynx is normal, but the oropharyngeal and hypopharyngeal airway is reduced in area by an average of 25% | Low |
| Cephalometric evaluation could be useful when used with head and neck examination, polysomnographic and endoscopic studies to evaluate OSA patients, and to assist with the planning/surgical treatment for improvement of upper airway patency | |||||
| Scannone, 2017 | Observational case control bi-variate analysis | 126 | Features of craniofacial and dental phenotype associated with high suspicion of OSA in adults using lateral cephalograms and study casts | Subjects with class II malocclusion more prone to OSA | Low |
| No other parameters showed any significant differences | |||||
| Kumar, 2007 | Observational | 10 | Comparison of orthogonal and prospective CBCT lateral projections | Orthogonal CBCT projections provided more accurate midsagittal skull measurements than prospective CBCT or conventional cephalometric radiographs | Low |
OSA, obstructive sleep apnea; AHI, apnea-hypopnea index; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; PSG, polysomnography; HSAT, home sleep apnea testing; ESS, Epworth Sleepiness Scale; UA-XSA, upper airway cross-sectional area; RDI, respiratory disturbance index; QUADAS, Quality Assessment of Diagnostic Accuracy Studies; ODI, oxygen desaturation index; O2 sat, oxygen saturation; LSAT, lowest oxyhemoglobin saturation; PSSD, portable sleep study device; CBCT, cone beam computed tomography.
Airway centric orthodontics: revising the clinical treatment paradigm
Much has been debated on early treatment, one phase or two-phase intervention. The reality remains that an essential amount of craniofacial growth is accomplished by 4 years of age. Almost complete development (90%) of the craniofacial complex is completed by 12 years of age and so it must follow that aberrant growth, morphologic features of sleep disordered breathing in adults may be a carryover from childhood issues that remain unaddressed both due to a lack of detail by the treating dentists and also the cognitive bias of treatment only once all the permanent teeth have erupted by a large segment of the lay population and the profession.
There is a need for orthodontists being sensitive to the reality of OSA as they play an important role in the diagnosis and management of OSA albeit as part of an interdisciplinary team.
OAT has emerged as one of the accepted modalities of management of OSA especially so in patients who do not tolerate the PAP devices (16), while early research has focused on OAT in patients with mild and moderate OSA (92) emerging evidence indicates that these devices could be beneficial to patients with severe OSA also especially in cases where CPAP has not been successful (93,94).
Comparisons of OAT with PAP devices have largely focused on the improvement in the AHI scores and thus OAT are often reported to be less efficacious in comparison to PAP for PSG parameters (95). In clinical practice, irrespective of the comparative measures used the better compliance and better health benefits achieved by OAT offset the inability of OAT to match the PAP devices in reducing apneic events (96).
Management of OSA in adults
Broadly OSA can be managed using three stratagems of behavioral modification, treatment devices and surgical intervention.
Drug therapy and behavioral modification
Drugs such as eszopiclone, acetazolamide, naltrexone, physostigmine, donepezil, and fluticasone have been tried out in the therapy of OSA, however there is insufficient scientific evidence to recommend the long-term use of these drugs in the treatment of OSA (97).
Behavioral modification including diet modifications (98) low energy diet (99) diet and exercise (100,101) have all been found to be beneficial in reducing the severity of OSA and achieving reduction of AHI scores (102). The findings of the articles quoted in this section are summarized in Table 6.
Table 6
| Author, year | Study type | Number of subjects | Outcome evaluated | Results and conclusion | Quality of evidence |
|---|---|---|---|---|---|
| Mason, 2013 | Systematic review | 30 trials | Efficacy of drug therapy in OSA management | Insufficient evidence for the use of drugs in the therapy of OSA | High |
| Papandreou, 2012 | Randomized trial | 940 | Effect of Mediterranean diet vs. prudent diet with exercise in obese OSA patients | Reduction in AHI during rapid eye movement sleep | Low |
| Johansson, 2011 | Single center prospective | 63 | To evaluate whether the initial effects of a low energy diet in OSA patients persist even after 1 year of the diet | Patients with severe OSA demonstrated marked reduction in AHI (reduction of 25/h) | Low |
| 30/63 did not require PAP after 1 year and 6/63 achieved complete remission | |||||
| Igelström, 2018 | Two arm RCT | 86 | Effect of behavioral sleep medicine on moderate and severe OSA | 40% of experimental group achieved improvement in OSA status | Moderate |
| Aiello, 2016 | Systematic review and meta-analysis | 8 articles | Efficacy of exercise in reducing AHI | Exercise as sole intervention led to better clinical outcomes | Low |
OSA, obstructive sleep apnea; AHI, apnea-hypopnea index; PAP, positive air pressure; RCT, randomized control trial.
Treatment with devices
The benchmark treatment for OSA continues to be CPAP and modifications such as BPAP, auto-titrating PAP (APAP), and humidified PAP (103). However, poor compliance is reported in around two-thirds of patients on PAP devices (6) and thus recent devices come with modifiable pressure profiles that result in greater patient compliance, however the compliance rates still continue to be an area of concern (16,104).
OAT
The oral cavity is an important component of the airway, the anterior boundary of the upper airway is formed by the upper and lower incisors and the piriform rim. The superior boundary is formed by the cranial base, the posterior boundary by the cervical vertebrae, and the inferior boundary is formed by the hyoid bone. The width of the palate, the separation between the ascending rami, and the middle cranial fossa are lateral determinants of airway size. The pharyngeal muscles, turbinates, tonsils, soft palate, tongue, adenoids, and nares line these structures which define the bony skeletal limits (67), Thus providing the dentists and orthodontists with opportunities to leverage these factors for the management of OSA. The challenge for the orthodontists is to address any craniofacial structural deformity that could directly or indirectly influence the airway and to look at the possibility of OAT in addressing OSA.
The first documented use of an oral appliance to improve respiration comes from Pierre Robin who in 1921 demonstrated the use of a monoblock appliance for a case of the Pierre Robin syndrome which essentially led to mandibular advancement with tongue and hyoid repositioning for the management of the Pierre Robin sequence (105).
There are over 100 types of oral appliances available for the treatment of OSA differing in terms of mechanism of action, location and materials used (106). These appliances can be grouped as follows:
- Palatal lift appliances/soft palate liners;
- Tongue retaining devices/tongue stabilizing devices (TRDs/TSDs);
- Mandibular advancement devices (MADs)/mandibular repositioning devices (MRDs)/mandibular advancing appliances (MAAs).
Drug-induced sleep endoscopy (DISE) a procedure fist recommended in 1991 to accurately locate the position of upper airway collapse during induced sleep is now being used to discern the value of OAT in patients who have failed CPAP or previous surgery (107,108).
Palatal lift appliances or soft palate liners
These were initially used for the treatment of snoring by applying pressure on both the soft palate and uvula thereby reducing palatal vibrations, this was also found to increase airway space and hence were also used for OSA treatment (105,109). However, these appliances are rarely seen in clinical use exclusively for the management of OSA (106). Several devices such as the Tübingen palatal plate used for the management of the Robin Sequence also improve OSA parameters (110).
TRDs/TSDs
TRD/TSDs work on the principle of clasping the tongue in a forward position and not allowing it to proceed downward because of negative inspiratory pressure thus maintaining airway patency. A variety of TRD designs are available and have shown to significantly reduce the AHI scores in over 71% of patients (111,112) and TRDs are ideal for use in edentulous patients and patients with unhealthy dentition in whom other OAT is precluded (113).
MADs
The American academy of dental sleep medicine in 2014 gave an evidence-based definition for an effective appliance in the treatment of OSA and snoring and have constructed the definition for MAD as they are the most effective and clinically widely used OAT appliance (114).
Titratable functional appliances have been the precursors to the MADs for adults, MADs as the name suggest reposition or advance the mandible and base of tongue leading to tightening of the supra hyoid, infrahyoid, genioglossus, and geniohyoid muscles causing an increase in pharyngeal airway space and resultant decrease in the PCRIT (115) an important consideration in MAD therapy is the amount of mandibular advancement possible since devices in which advancement is not beyond centric occlusion do not effect airway size and in general the degree of mandibular attachment dictates the efficacy of MAD devices (105). MADs have generally shown optimum results at a 50% protrusion and protrusion beyond 50% has not shown any added benefits, the effect of less than 50% protrusion is not yet clearly documented (116,117).
Boil and bite devices
The simplest MAD device available are the boil and bite devices such as the Sleep Pro 1 and SnoreRx consist of a thermoplastic material which can be heated and the patient bites on these blocks and allows the material to set. The boil and bite devices suffer from improper fit and fall out easily, nevertheless these devices have also been found to improve AHI scores (118).
Customized MADs
Customized MADs are better tolerated than the prefabricated boil and bite (119), devices and come in two models, the one piece or monobloc appliances and the two piece or duo bloc appliances which consist of two blocks connected to each other, the two piece appliances come with the added benefit of being adjustable serially titrable i.e., the mandibular protrusion in these devices can be adjusted gradually allowing the patient to slowly adjust to maximum protrusion in contrast to the monobloc devices. Isacsson et al. have reported similar efficacy rates in their multicentric trial and Ishiyama et al. found better results for the monoblock devices in their meta-analysis (120-122).
The advent of digital impressions, computer-aided design (CAD)/computer-aided manufacturing (CAM) and 3D printing have led to the emergence of several Food and Drug Administration (FDA) approved MAD appliances such as Narval, Pantera, and Somnodent avant which are 3D printed and the Prosomnus Micro2 which is a CAD/CAM milled appliance (Figure 5) (123), these come with the benefits of being less bulky and have a better fit and have also shown a good response in severe cases of OSA (117,124).
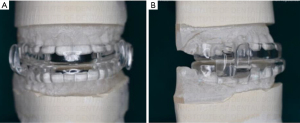
The MAD appliances are usually delivered with the mandible obtruded to two-thirds of its maximal protrusion (Figure 6). A second sleep study after the patient is well accommodated and has some relief would be an indicator of success of the therapy. Monitoring every 6 months and then annually after the first year is recommended in various guidelines (113).
Selection of patients for OAT
The key to successful management of OSA by OAT would be appropriate patient selection. A stable dentition, minimum of 6–8 teeth/arch, good oral and periodontal health are prerequisites for a patient to be subjected to MAD appliances, though no absolute contraindications exist the presence of loose crowns, pre-existing temporomandibular disorders (TMDs), bruxism, micrognathia are relative contraindications (106,120).
Patients likely to benefit from MAD appliances are likely to be younger females, patients with a lower BMI and smaller neck circumference (120).
Efficacy of OAT
In consonance with the WHO definition of health the authors believe that the efficacy of OAT should be assessed on the basis of improvement in the quality of life indicators. Narratives of OSA efficacy have largely been affected by comparisons to CPAP and physiological indicators and the AHI index (106).
There exist ample evidence from several randomized control trials (RCTs) for the use of OAT in OSA especially in patients not compliant with PAP devices (97,98,107,112,125-131), this evidence is further supplemented by several systematic reviews and meta-analysis (112,116,117,122,130-133). all of these have reflected on the fact that OAT and PAP devices provide comparable health outcomes, with PAP devices showing a greater reduction in OSA severity as assessed by PSG.
The systemic effects of OAT have also been widely studied for over 2–8 years with OAT bringing about modest but significant benefits on cardiovascular outcomes, blood pressure, cognitive function, depressive symptoms, headache and quality of life (93,126,134-138).
Adverse effects of OAT
Temporomandibular joint (TMJ) discomfort or pain is the most common complaint of patients being treated with MADs occurring in over 37% of patients after a year of use according to a questionnaire study (139).
Increased salivation, dry mouth, bad taste in the mouth and occlusal changes have also been reported (118,140). Alterations in cephalometric measurements have also been found post use of MAD devices (141,142). The results of various studies quoted in this section are summarized in Table 7.
Table 7
| Author, year | Study type | Number of subjects | Outcome evaluated | Results and conclusion | Quality of evidence |
|---|---|---|---|---|---|
| Marklund, 2017 | Literature review | 102 articles | Evidence for OAT in the treatment of OSA | High level of evidence shows that OTA is effective in the treatment of OSA, but CPAP is more efficient. Higher adherence with OTAs may compensate for this difference | Low |
| Gjerde, 2016 | Single center retrospective 2007–2013 | 116 | To test the efficacy of MADs in 116 patients with moderate and severe OSA who were non-compliant with CPAP | No significant difference between the AHI reductions achieved by MADs in moderate and severe OSA | High |
| Sutherland, 2015 | Multicentric retrospective | 425 | Comparison of a large cohort of MAS treated patients (I) to compare efficacy across patients with different phenotypes of OSA and (II) to assess demographic, anthropometric, and PSG variables | Lower MAS treatment response rates were observed in supine and REM sleep | Moderate |
| Demographic and anthropometric and PSG variables weakly inform about MAD efficiency | |||||
| Barthlen, 2000 | Single center prospective | 24 | Comparison of MAD, TRD and soft palate lift appliance in OSA management | 8/8 complied with MAD therapy whereas only 5/8 and 2/8 with TRD and soft palate lift respectively | Low |
| Lazard, 2009 | Retrospective single center | 84 | Efficacy and tolerance of TRDs in patients with OSA | TRDs show similar efficacy and tolerance as MADs | Low |
| Ferguson, 1998 | Single center prospective | 19 | Videoendoscopic measurement of upper-airway cross-sectional area shape in the hypopharynx, oropharynx, and velopharynx during various stages of active mandibular and tongue protrusion | Both protrusions increased upper-airway cross section with tongue protrusion causing more oropharynx and velopharyngeal increase and mandibular protrusion causing increase in hypopharynx | Low |
| Quinell, 2019 | Randomized control crossover trial | 90 | To compare clinical- and cost-effectiveness of a range of MADs against no treatment in mild to moderate OSA | All MAD devices used reduced AHI | High |
| Non-adjustable MADs achieve clinically important improvements in mild to moderate OSA and are cost-effective | |||||
| The semi customized MAD is an appropriate first choice | |||||
| Isacsson, 2019 | Multicenter randomized trial | 302 | The patients were randomized to monobloc and bibloc devices and effect on reduction of AHI was compared | Both devices achieved a mean reduction of 12–14 AHI events/h | High |
| Aziz, 2021 | Single center prospective | 10 | Efficacy of CAD/CAM milled appliance in management of OSA as assed by cephalometric measurements and ESS | Increase in pharyngeal airway width, velum dimension and anterosuperior repositioning of mandible | Low |
| All patients demonstrated decreased ESS scores | |||||
| Comparison of OAT with CPAP | |||||
| Schütz, 2013 | Single center prospective | 25 | Effect of CPAP, OAT and continuous exercise training on OSA | Only CPAP and OAT were able to bring about significant reductions in AHI (P=0.01) with only CPAP bringing about normalization of AHI | Low |
| Doff, 2013 | Cohort study [2002–2005] | 103 | Comparison of objective (PSG) and objective (ESS, short form health survey-36, functional outcomes of sleep questionnaire) | No significant difference was found between OAT and CPAP in treating mild to severe OSAs in a 2-year follow up | High |
| Both therapies showed significant improvements in PSG and neurobehavioral outcomes | |||||
| CPAP more effective in reducing AHI (P<0.05) | |||||
| OAT is a viable alternative to CPAP in mild to moderate OSA. For severe OSA CPAP is the first line of treatment | |||||
| Ferguson, 1996 | Randomized, prospective, cross over | 25 | To compare efficacy, side effects, patient compliance, and preference between OAT and CPAP | AHI reduction with CPAP (3.5) was better in comparison with OAT (9.7) (P<0.05) | Moderate |
| Of the seven patients who were treatment success with both treatments 6 preferred OAT as long-term therapy | |||||
| OAT is effective and is associated with lesser side effects and has greater patient satisfaction than CPAP | |||||
| Nikolopoulou, 2017 | Randomized, placebo-controlled trial | 64 | Comparison the effects of a MAD with those of CPAP on self-reported symptoms of common sleep disorders and sleep-related problems in mild and moderate OSA patients | The three groups (MAD, CPAP, and placebo) showed significant improvements over time in symptoms corresponding with ‘insomnia’, ‘excessive daytime sleepiness’, ‘psychiatric sleep disorder’, ‘periodic limb movements’, ‘sleep apnoea’, ‘sleep paralysis’, ‘daytime dysfunction’, ‘hypnagogic hallucinations/dreaming’, ‘restless sleep’, ‘negative conditioning’ and ‘automatic behaviour’ (range of P values: 0.090–0.897) | Moderate |
| Dort, 2008 | Randomized controlled crossover | 38 | Evaluation of a non-customized TRD compared to a control device as assessed by a reduction in RDI | The customized TRD brought about a significant reduction in RDI (P=0.06) | Low |
| The customized TRD was better preferred by 54% patients | |||||
| Comparison of OAT with CPAP literature reviews and meta-analysis | |||||
| Poets, 2019 | Literature review | – | Efficacy of Tübingen plate in the management of Robin sequence and resultant upper airway obstruction | The Tübingen plate was superior to a sham procedure in reducing upper airway obstruction on patients with Robin sequence | Low |
| Chang, 2017 | Systematic review and meta-analysis | 16 studies and 242 patients | TRD outcomes as a treatment of OSA | Current international literature demonstrates that TRDs reduce | Moderate |
| AHI by 53%, increase lowest oxygen saturation by 4.1 oxygen saturation points, decrease oxygen desaturation index by 56% and decrease ESS scores by 2.8 points | |||||
| TRDs provide a statistically effective alternative treatment option for OSA | |||||
| Bartolucci, 2016 | Systematic review and meta-regression analysis | 13 RCTs | Effectiveness of different MADs in reducing AHI in OSA patients | Mandibular advancements from 50–89% | Moderate |
| Advancement amounts greater than 50% do not significantly increase success rates (P=0.541) | |||||
| AHI improvement not proportional to mandibular advancement | |||||
| Li, 2020 | Systematic review and meta-analysis | 14 RCTs 249 patients | Efficacy of CPAP vs. MAD in the treatment of OSA | CPAP was significant in reducing AHI (7.09/h) | Moderate |
| CPAP has better therapeutic efficacy | |||||
| Rangarajan, 2022 | Systematic review and meta-analysis | 25 studies | Impact of OAT on quality of life in patients with OSA assessed by functional outcomes of sleep questionnaire | With a mean difference of 1.8 points between pre and post therapy scores OAT treatment led to significant improvement in quality of life | Low |
| Ilea, 2019 | Systematic review | 15 | Effectiveness of MAD for OSA by improvement of AHI | 92% of subjects successfully treated with OAT | Low |
| Ishiyama, 2019 | Systematic review and meta-analysis | 7 RCTs | Efficacy of device designs (monoblock or biblock) in MAD for OSA patients | Monoblock significantly reduced AHI in comparison to bi block (P=0.0006) and had better patient preference (P=0.0001) | Moderate |
| MAD effects on systemic health | |||||
| Kim, 2022 | Literature review | 41 articles | To present an overview of the cur-rent evidence regarding the overall multisystemic effects of MAD in OSA patients | MAD reduce diastolic blood pressure, heart rate and heart rate variability | Low |
| Levels of cardiovascular markers IL-6 and TNF-α | |||||
| MAD brings about significant improvements in glycated hemoglobin levels in moderate OSA but not severe OSA patients | |||||
| Improvement in vigilance and psychomotor speed | |||||
| Yoshida, 2006 | Single center prospective | 194 | Evaluation the effect of individually prescribed oral appliances for the treatment of OSA syndrome | OAT resulted in a reduction of mean blood pressure 3.7 mmHg with maximum effect seen in the diastolic of 4.5 mmHg (P<0.001) | Low |
| The reduction was more in responders to OAT (higher reduction in AHI) | |||||
| Trzepiur, 2009 | Single center prospective case control | 33 | Impact of CPAP and MAD on microvascular endothelial function using laser doppler flowmetry | Significant increase in acetylcholine induced dilatations (P=0.016) ensuring better nocturnal oxygenation | Low |
| Galic, 2016 | Single center prospective | 18 | Effect of MAD treatment on arterial stiffness, glucose metabolism, and certain inflammatory markers as predictors of cardiometabolic risk in mild to moderate OSA patients | MAD resulted in reduced plasma fasting glucose levels after 1 year (P<0.01) | Low |
| Reduction in inflammatory marker plasma fibrinogen after 1 year (P<005) | |||||
| Long-term effects of MAD | |||||
| Haviv, 2014 | Single center retrospective 2006–2012 | 52 | Evaluation of medium long-term outcome and success rates of oral appliances in patients with severe OSA by second PSG 2 years after initiation of MAD therapy | A significant reduction (P<0.0001) in the AHI was demonstrated between the initial somnography (55.25) and follow up (10.79) | |
| Marklund, 2007 | Single center retrospective 2004–2007 | 260 | Questionnaire based study with mean follow-up of 5.4 years | Frequent use (P=0.001), few night-time awakenings before start of treatment (P=0.02), and effective apnea reduction during treatment of more severe cases (P=0.02) correlated with a good subjective effect at long-term follow-up | Moderate |
| Doff, 2013 | Cohort study [2002–2005] | 103 | Comparison of objective (PSG) and objective (ESS, short form health survey-36, functional outcomes of sleep questionnaire) | No significant difference was found between OAT and CPAP in treating mild to severe OSAs in a 2-year follow up | High |
| Adverse effects of MAD | |||||
| Pliska, 2014 | Single center retrospective 10-year follow-up | 77 | Evaluate the magnitude and progression of dental changes associated with long-term MAS treatment | Significant (P<0.001) reduction in the overbite (2.3±1.6 mm), overjet (1.9±1.9 mm), and mandibular crowding (1.3±1.8 mm) | High |
| A corresponding significant (P<0.001) increase of mandibular intercanine (0.7±1.5 mm) and intermolar (1.1±1.4 mm) width as well as incidence of anterior crossbite and posterior open bite was observed | |||||
| McGown, 2001 | Questionnaire survey | 166 | To collect subjective and objective information concerning long-term effects, compliance and side effects of MAD devices | TMJ pain was the most common adverse effect (26/69), discomfort (25/69), sleep disturbance (12/69), excessive salivation (7/69), and altered bite (9/69) were noted as adverse effects | Low |
| Marklund, 2006 | Single center prospective questionnaire survey 5.4-year follow up | 450 | Find predictors of dental side effects from monoblock MADs after a 5.4-year follow-up using questionnaire and cast measurements | Median reductions of overjet and overbite of 0.6 mm after 5 years of MAD use | Moderate |
| De Almeida, 2006 | Single center prospective | 71 | Patients were evaluated for adverse effects using lateral cephalometric measurements after 7.3±2.1 years of MAD use | Significant (P<0.01) in the following variables: increase in mandibular plane and ANB angles; decrease in overbite and overjet; retroclined maxillary incisors; proclined mandibular incisors; increased lower facial height; and distally tipped maxillary molars with mesially tipped and erupted mandibular molars | Moderate |
| De Almeida, 2006 | Single center prospective | 71 | Patients were evaluated for adverse effects using model analysis after 7.3±2.1 years of MAD use | 14.3% had no occlusal changes, of the 85.7% who had changes 41.4% had favorable changes, and 44.3% had unfavorable changes | Moderate |
| Class II malocclusions are likely to have favorable changes | |||||
OAT, oral appliance therapy; OSA, obstructive sleep apnea; CPAP, continuous positive air pressure; MAD, mandibular advancement device; AHI, apnea-hypopnea index; MAS, macrophage activation syndrome; PSG, polysomnography; REM, rapid eye movement; TRD, tongue retaining device; CAD, computer-aided design; CAM, computer-aided manufacturing; ESS, Epworth Sleepiness Scale; RDI, respiratory disturbance index; RCT, randomized control trial; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α; TMJ, temporomandibular joint; ANB, A-point-nasion-B-point.
Surgical management
Surgical interventions in adult OSA are never the first line of treatment and are only considered after other modalities have failed or cannot be implemented (143). The cases need careful evaluation and possible impact on airway and alleviation of symptoms.
Nasal surgeries such as septoplasty (with or without turbinate reduction) have been found to reduce sleepiness in a RCT (144). Tonsillectomies are recommended for adults with tonsillar hyperplasia although evidence of contribution of tonsillar hyperplasia to adult OSA is still unclear (145).
Oropharyngeal surgeries such as uvulopalatopharyngoplasty and modifications such as laser-assisted uvulopalatopharyngoplasty, radiofrequency palatoplasty, Z palatoplasty involve reduction of palatal bulk by removal of uvula, part of soft palate and tonsil and closure of tonsillar pillars (146). These are the commonest surgical procedures being performed for adult OSA with success rates of 50% and cure rates of 16% (147).
Procedures on the tongue such as reduction glossectomies have been found to improve quality of life while reducing daytime sleepiness (147), and have provided variable success rates, ranging from 35% to 62% (148).
Maxillomandibular advancement (MMA) surgeries involve osteotomies (Le Fort 1 and bilateral mandibular ramus sagittal split) of the maxilla and mandible followed by counter clockwise advancement of the inferior portion of the maxilla and anterior mandible leading to a forward displacement of attached soft tissues, tensioning the pharynx with resultant increase of the pharyngeal air space (146,149). These procedures also lead to better facial aesthetics (150,151). MMA surgeries demonstrate a high success rate (86–90%) and are the only treatment modalities demonstrating cure rates similar to CPAP (152). MMA surgeries are usually done as part of a multilevel or staged surgeries with stage 1 consisting of soft tissue surgeries and MMA as stage 2 surgeries (143).
Surgically assisted rapid palatal expansion (SARPE) and micro implant assisted rapid palatal expansion (MARPE) are modalities for increasing the transverse maxillary dimensions in individuals with transverse deficiency (153). This expansion has the secondary effect of decreasing airway resistance and increasing airway space. Both modalities have been found to decrease rate of respiratory disturbances in OSA (154).
Genial advancement, tracheostomy and surgical implantation of a hypoglossal nerve stimulation device that has been deemed safe, feasible and efficacious in two trials are the other surgical procedures used in adult OSA (155,156). Table 8 summarizes this section.
Table 8
| Author, year | Study type | Number of subjects | Outcome evaluated | Results and conclusion | Quality of evidence |
|---|---|---|---|---|---|
| Koutsourelakis, 2008 | Single center randomized trial | 49 | Post-surgical increase in nasal epoch leading to AHI change in comparison with a sham surgery assessed via breathing route examination | 14.8% of surgical group responded to surgery with considerable increase in nasal epochs | Low |
| Nasal surgery rarely treats OSA effectively | |||||
| Kezirian, 2006 | Literature review | 36 articles | Evidence-based medicine review of the literature describing outcomes of hypopharyngeal surgery in OSA | Improvements in respiratory physiology during sleep, daytime somnolence, and quality of life | Low |
| Vicini, 2010 | Single center RCT | 56 | Random allocations of 50 consecutive patients to APAP or surgical management (MMA) and comparison of demographics, biometric parameters, ESS, and AHI | Significant improvement of AHI and ESS scores | Moderate |
| No differences between the two groups (P=0.20) | |||||
| Oliveira, 2021 | Single center prospective | 17 | Comparison of skeletal and dental changes of MARPE vs. SARPE using CBCT | MARPE showed an increase in skeletal transverse maxillary expansion at the palate and basal bone compared with SARPE, especially at the posterior region. No difference was found in the expansion of the alveolar process between the two methods | Low |
| MARPE presented a more parallel expansion in both the coronal and axial view, whereas SARPE resulted in a more triangular and V-shaped opening | |||||
| A greater buccal inclination of the alveolar process and supporting teeth was observed in the SARPE group | |||||
| Vinha, 2016 | Single center prospective study | 16 | Effects of SARPE on PSG parameters in patients with transverse maxillary deficiency | A RDI reduction of 54.6% (P=0.0013) | Low |
| A 56.2% (P=0.001) decrease was found in the AHI, in addition to decreases in the desaturation and microarousal rates, among other parameters | |||||
| The ESS scores improved from 12.5±5.3 to 7.2±3.5 | |||||
| Oliven, 2011 | Literature review | – | Review of hypoglossal nerve stimulation in OSA management | 50% reduction in AHI score achieved by hypoglossal nerve stimulation devices | Low |
| Hypoglossal nerve stimulation devices likely to be available as a treatment option soon | |||||
| Kezirian, 2006 | Single center prospective study | 31 | Examine the safety, feasibility, and efficacy of a novel HGNS system (HGNS®, Apnex Medical, Inc., St. Paul, MN, USA) in treating OSA at 12 months following implantation | Significant improvement (P<0·001) from baseline to 12 months in AHI (45.4±17.5 to 25.3±20.6 events/h) | Low |
| No major adverse effects alluding to the safety of HGNS |
OSA, obstructive sleep apnea; AHI, apnea-hypopnea index; RCT, randomized control trial; APAP, auto-titrating positive air pressure; MMA, maxillomandibular advancement; ESS, Epworth Sleepiness Scale; MARPE, micro implant assisted rapid palatal expansion; SARPE, surgically assisted rapid palatal expansion; CBCT, cone beam computed tomography; PSG, polysomnography; RDI, respiratory disturbance index; HGNS, hypoglossal nerve stimulation.
OSA in children
Pediatric OSA is pathogenetically different from adult OSA because of the role played by multiple risk factors such as airway collapse, impaired neuromuscular tone, hyperplasia of tonsils and surrounding lymphoid tissue and growth-related changes contribute to pediatric OSA. Childhood obesity is a growing concern, and any screening must consider this essential aspect (157). According to the International Classification of Sleep Disorders (ICSD)3 (158), OSA can be diagnosed by either of two sets of diagnostic criteria. The first set of criteria for OSA includes the presence of at least one of the following: (I) snoring; (II) labored, paradoxical, or obstructed breathing during the child’s sleep; or (III) sleepiness, hyperactivity, behavioral problems, or learning problems. The PSG criterion for diagnosis requires either (I) one or more obstructive events (obstructive or mixed apnea or obstructive hypopnea) per hour of sleep or (II) obstructive hypoventilation, manifested by PaCO2 >50 mmHg for >25% of sleep time, coupled with snoring, paradoxical thoracoabdominal movement, or flattening of the nasal airway pressure waveform. It would be necessary to emphasize that these criteria are for use only in children under 18 years, though for ages 13–18 years the adult criteria may also be used (17). The pediatric sleep questionnaire is recommended as an adjunct to PSG analysis (159). In addition, HSATs with oximetry, DISE and dynamic MRI are used in diagnosis of pediatric OSA (160).
Management of pediatric OSA
Management of pediatric OSA differs from adult OSA in a multitude of ways, surgical management gains primary importance with adenotonsillectomy becoming the gold standard surgical treatment, with a metanalysis demonstrating resolution of PSG findings in 83% of patients (161). In children with deficient jaw size or with syndromes such as Pierre Robin sequence and trencher Collin syndrome MMA surgeries have proven to be beneficial (157). In cases where MMA surgeries cannot provide the desired increase in space, distraction osteogenesis is emerging as a viable alternative as the gradual maxillary/mandibular advancement provides for better soft tissue adaptation. The decreased PSG parameters are stable in the long run (17,161).
MAD appliances are when used in children will stimulate cartilaginous growth at the condyles and a recent metanalysis has found MAD devices in children to be most effective when used before the pubertal peak (162). In cases of transverse nasomaxillary deficiency the use of rapid maxillary expansion (RME) or MARPE has been found to reduce AHI scores in the short-term while there is insufficient long-term data. These devices bring about a transverse expansion of the palate, maxillary base and nasal floor with a downward shift of the nasomaxillary complex thereby decreasing airway resistance and increasing airway space (163,164).
Increased vertical face dimension is one of the classic findings in patients with OSA, this is largely caused by a clockwise rotational growth of the, to counteract this a vertical pull headgear with intraoral maxillary micro implants favoring a counter clockwise mandibular growth with concomitant reduction in vertical facial height (165) has also been tried with mixed results precluding any recommendations (166).
The findings of this review on pediatric OSA are summarized in Table 9.
Table 9
| Author, year | Study type | Number of subjects | Outcome evaluated | Results and conclusion | Quality of evidence |
|---|---|---|---|---|---|
| Brietzke, 2006 | Meta-analysis | 14 studies | Effectiveness of adenotonsillectomy in treating obstructive sleep OSA in uncomplicated pediatric patients | The mean sample size was 28 | Moderate |
| Reduction of 13.92 AHI events/h (P=0.001) | |||||
| Success rate of procedure was 82.9% | |||||
| Yanyan, 2019 | Systematic review and meta-analysis | 7 studies | Evaluation of the effect of MAA in pediatric OSA | Mean reduction of 1.75 AHI events/h (P=0.0001) noted | Moderate |
| MAA can be effective for mild to severe patients before the end of the pubertal peak | |||||
| Long-term treatment (at least 6 months) may be more effective than short-term treatment | |||||
| Bucci, 2023 | Systematic review and meta-analysis | 25 studies | The effect of the following five interventions: RME, MAA, MT, and RME combined with MAA in bringing about changes in AHI and SaO2 | Significant reductions of AHI achieved by RME and MAA at 6 months to 1 year after start of therapy | Moderate |
| Myofunctional therapy cannot be suggested as an elective treatment because of lack of quality evidence | |||||
| Comacho, 2016 | Systematic review and meta-analysis | 17 studies | Analysis of sleep study outcomes in children who have undergone RME as treatment for OSA | 70% of patients showed decreased in AHI (reduction of 6 events/h) | Moderate |
| Increase of oxygen saturation from 87 to 96 | |||||
| Improvements marked in patients who have undergone adenotonsillectomy | |||||
| Shavakhi, 2019 | Systematic review and meta-analysis | 7 studies | Effect of headgear on airway dimensions in patients with class II malocclusion | Low quality of evidence | Low |
| Conclusions of articles differed from each other |
OSA, obstructive sleep apnea; AHI, apnea-hypopnea index; MAA, mandibular advancement; RME, rapid maxillary expansion; MT, myofunctional therapy; SaO2, arterial oxygen saturation.
Strengths
This review charts the spectrum of OSA from its evolutionary basis to the biological effects, diagnosis and treatment of the condition from the perspective of orthodontists.
Limitations
Being a literature review in the context of OSA and orthodontics not all aspects of OSA have been discussed. The review has utilized literature published in the English language only and hence data from other language articles are not included. The literature search may have missed out on a few relevant publications.
Conclusions
Airway centric orthodontics underlines a major shift from the tooth paradigm to a larger definition of health and well-being. OSA is major medical disorder that can have serious health consequences in both children and adults. An anthropologic predisposition consequent to bipedalism, phonetics, and the erect posture of homo sapiens has been further compounded by a progressive reduction in the size of the jaws, malocclusions, altered dietary patterns, obesity and societal change. There exists a need to screen all pediatric and adult patients for OSA in an orthodontic setting, a simple questionnaire procedure can be incorporated into the initial history taking and record keeping. Though diagnosis of OSA is based on PSG/HSAT parameters, the orthodontist would be able to identify the altered skeletal morphology which could impede the airway. OAT therapy achieves the alteration of mandibular/tongue position affecting airway dynamics and lowering the PCRIT. Surgical correction of maxillomandibular bases becomes essential in extreme cases. Maxillary expansion by SARPE/MARPE/RME/distraction osteogenesis could alter the nasal airway volume and the resistance to airflow.
OAT has emerged as a treatment of choice in the management of OSAs with several RCTs demonstrating the utility of OAT. This is especially in patients who are not amenable to PAP devices with MADs being the most common and effective devices available to orthodontists to manage OSA.
Slowly but surely a bridge is being built between orthodontics, oral medicine, oral, surgery and sleep medicine leading to the creation of multidisciplinary teams which will serve the specialty of dentistry well in enhancing its image rather than being relegated to a niche segment of cosmetics and aesthetics alone. It’s time to redefine the paradigm.
Acknowledgments
The authors acknowledge Medeva Knowledge Systems, New Delhi, and Dr. Uday for the AI-Based Literature Search and Collation, and acknowledge Dr. Goutham Reddy & Dr. Rezin Aziz, Department of Orthodontics, Coorg Institute of Dental Sciences for data from their dissertation on “Comparative assessment of changes in pharyngeal airway space in cases of obstructive sleep apnoea with a customized mandibular repositioning appliance—a clinical study”.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Chung H. Kau) for the series “Managing Craniofacial and Dentofacial Disorders—Simplified Solutions for Difficult Situations” published in Frontiers of Oral and Maxillofacial Medicine. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://fomm.amegroups.com/article/view/10.21037/fomm-23-1/coif). The series “Managing Craniofacial and Dentofacial Disorders—Simplified Solutions for Difficult Situations” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patients for publication of their images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fietze I, Laharnar N, Obst A, et al. Prevalence and association analysis of obstructive sleep apnea with gender and age differences - Results of SHIP-Trend. J Sleep Res 2019;28:e12770. [Crossref] [PubMed]
- Myers KA, Mrkobrada M, Simel DL. Does this patient have obstructive sleep apnea?: The Rational Clinical Examination systematic review. JAMA 2013;310:731-41. [Crossref] [PubMed]
- Martin SA, Appleton SL, Adams RJ, et al. Nocturia, Other Lower Urinary Tract Symptoms and Sleep Dysfunction in a Community-Dwelling Cohort of Men. Urology 2016;97:219-26. [Crossref] [PubMed]
- Russell MB, Kristiansen HA, Kværner KJ. Headache in sleep apnea syndrome: epidemiology and pathophysiology. Cephalalgia 2014;34:752-5. [Crossref] [PubMed]
- Yue HJ, Bardwell W, Ancoli-Israel S, et al. Arousal frequency is associated with increased fatigue in obstructive sleep apnea. Sleep Breath 2009;13:331-9. [Crossref] [PubMed]
- Gottlieb DJ, Punjabi NM. Diagnosis and Management of Obstructive Sleep Apnea: A Review. JAMA 2020;323:1389-400. [Crossref] [PubMed]
- Stelzer FG, Garcia E, Schorr F, et al. Prevalence of chronic insomnia in patients with obstructive sleep apnea. Braz J Psychiatry 2021;43:370-5. [Crossref] [PubMed]
- Dempsey JA, Veasey SC, Morgan BJ, et al. Pathophysiology of sleep apnea. Physiol Rev 2010;90:47-112. [Crossref] [PubMed]
- Rundo JV. Obstructive sleep apnea basics. Cleve Clin J Med 2019;86:2-9. [Crossref] [PubMed]
- Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med 2019;7:687-98. [Crossref] [PubMed]
- Veasey SC, Rosen IM. Obstructive Sleep Apnea in Adults. N Engl J Med 2019;380:1442-9. [Crossref] [PubMed]
- Senaratna CV, Perret JL, Lodge CJ, et al. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med Rev 2017;34:70-81. [Crossref] [PubMed]
- Miyao E, Noda A, Miyao M, et al. The role of malocclusion in non-obese patients with obstructive sleep apnea syndrome. Intern Med 2008;47:1573-8. [Crossref] [PubMed]
- Singh H, Sharma P, Kapoor P, et al. Role of Malocclusion and Craniofacial Morphology in Obstructive Sleep Apnea. Indian Sleep Med 2019;14:10-7. [Crossref]
- Chang HP, Chen YF, Du JK. Obstructive sleep apnea treatment in adults. Kaohsiung J Med Sci 2020;36:7-12. [Crossref] [PubMed]
- Patil SP, Ayappa IA, Caples SM, et al. Treatment of Adult Obstructive Sleep Apnea With Positive Airway Pressure: An American Academy of Sleep Medicine Systematic Review, Meta-Analysis, and GRADE Assessment. J Clin Sleep Med 2019;15:301-34. [Crossref] [PubMed]
- Kim SJ, Ahn HW, Kim SW. Advanced interdisciplinary treatment protocol for pediatric obstructive sleep apnea including medical, surgical, and orthodontic care: a narrative review. Cranio 2023;41:274-86. [Crossref] [PubMed]
- Lyons-Coleman M, Bates C, Barber S. Obstructive sleep apnoea and the role of the dental team. Br Dent J 2020;228:681-5. [Crossref] [PubMed]
- Bauça JM, Yañez A, Fueyo L, et al. Cell Death Biomarkers and Obstructive Sleep Apnea: Implications in the Acute Coronary Syndrome. Sleep 2017; [PubMed]
- Morrell MJ, Finn L, Kim H, et al. Sleep fragmentation, awake blood pressure, and sleep-disordered breathing in a population-based study. Am J Respir Crit Care Med 2000;162:2091-6. [Crossref] [PubMed]
- Chen Y, Chen Y, Wen F, et al. Does continuous positive airway pressure therapy benefit patients with coronary artery disease and obstructive sleep apnea? A systematic review and meta-analysis. Clin Cardiol 2021;44:1041-9. [Crossref] [PubMed]
- Resta O, Foschino Barbaro MP, Bonfitto P, et al. Hypercapnia in obstructive sleep apnoea syndrome. Neth J Med 2000;56:215-22. [Crossref] [PubMed]
- Wang XN, Luo JM, Xiao Y, et al. Daytime hypercapnia in adult patients with obstructive sleep apnea in China. Chin Med J (Engl) 2021;134:2237-9. [Crossref] [PubMed]
- Kahn S, Ehrlich PR. Jaws: The story of a hidden epidemic. 1st ed. Redwood City: Stanford University Press; 2018.
- Kahn S, Ehrlich P, Feldman M, et al. The Jaw Epidemic: Recognition, Origins, Cures, and Prevention. Bioscience 2020;70:759-71. [Crossref] [PubMed]
- Lieberman DE, Krovitz GE, Yates FW, et al. Effects of food processing on masticatory strain and craniofacial growth in a retrognathic face. J Hum Evol 2004;46:655-77. [Crossref] [PubMed]
- GOOSE DH. Reduction of palate size in modern populations. Arch Oral Biol 1962;7:343-50. [Crossref] [PubMed]
- Corruccini RS. An epidemiologic transition in dental occlusion in world populations. Am J Orthod 1984;86:419-26. [Crossref] [PubMed]
- Bresolin D, Shapiro PA, Shapiro GG, et al. Mouth breathing in allergic children: its relationship to dentofacial development. Am J Orthod 1983;83:334-40. [Crossref] [PubMed]
- Harari D, Redlich M, Miri S, et al. The effect of mouth breathing versus nasal breathing on dentofacial and craniofacial development in orthodontic patients. Laryngoscope 2010;120:2089-93. [Crossref] [PubMed]
- D'Onofrio L. Oral dysfunction as a cause of malocclusion. Orthod Craniofac Res 2019;22:43-8. [Crossref] [PubMed]
- Harvold EP, Tomer BS, Vargervik K, et al. Primate experiments on oral respiration. Am J Orthod 1981;79:359-72. [Crossref] [PubMed]
- Moreno Uribe LM, Miller SF. Genetics of the dentofacial variation in human malocclusion. Orthod Craniofac Res 2015;18:91-9. [Crossref] [PubMed]
- Mew JR. The postural basis of malocclusion: a philosophical overview. Am J Orthod Dentofacial Orthop 2004;126:729-38. [Crossref] [PubMed]
- Engelke W, Jung K, Knösel M. Intra-oral compartment pressures: a biofunctional model and experimental measurements under different conditions of posture. Clin Oral Investig 2011;15:165-76. [Crossref] [PubMed]
- Cao Y, Ning P, Li Q, et al. Cancer and obstructive sleep apnea: An updated meta-analysis. Medicine (Baltimore) 2022;101:e28930. [Crossref] [PubMed]
- Li X, Liu Y, Rich SS, et al. 0291 Sleep Disordered Breathing Associated with Epigenetic Age Acceleration: Evidence from the Multi-Ethnic Study of Atherosclerosis. Sleep 2019;42:A118-9. [Crossref]
- Xiong H, Lao M, Zhang S, et al. A cross-sectional study of obstructive sleep apnea in patients with colorectal cancer. J Gastrointest Oncol 2022;13:683-94. [Crossref] [PubMed]
- Blask DE. Melatonin, sleep disturbance and cancer risk. Sleep Med Rev 2009;13:257-64. [Crossref] [PubMed]
- Wong ATY, Heath AK, Tong TYN, et al. Sleep duration and breast cancer incidence: results from the Million Women Study and meta-analysis of published prospective studies. Sleep 2021;44:zsaa166. [Crossref] [PubMed]
- Shiomi T, Guilleminault C, Stoohs R, et al. Leftward shift of the interventricular septum and pulsus paradoxus in obstructive sleep apnea syndrome. Chest 1991;100:894-902. [Crossref] [PubMed]
- Leuenberger UA, Hardy JC, Herr MD, et al. Hypoxia augments apnea-induced peripheral vasoconstriction in humans. J Appl Physiol (1985) 2001;90:1516-22. [Crossref] [PubMed]
- Xie A, Skatrud JB, Morgan B, et al. Influence of cerebrovascular function on the hypercapnic ventilatory response in healthy humans. J Physiol 2006;577:319-29. [Crossref] [PubMed]
- Weitzenblum E, Chaouat A. Obstructive sleep apnea syndrome and the pulmonary circulation. Ital Heart J 2005;6:795-8. [PubMed]
- El Solh AA, Akinnusi ME, Baddoura FH, et al. Endothelial cell apoptosis in obstructive sleep apnea: a link to endothelial dysfunction. Am J Respir Crit Care Med 2007;175:1186-91. [Crossref] [PubMed]
- Peres BU, Hirsch Allen AJ, Daniele P, et al. Circulating levels of cell adhesion molecules and risk of cardiovascular events in obstructive sleep apnea. PLoS One 2021;16:e0255306. [Crossref] [PubMed]
- Strohl KP, Boehm KD, Denko CW, et al. Biochemical morbidity in sleep apnea. Ear Nose Throat J 1993;72:34-39-41. [Crossref] [PubMed]
- Al-Delaimy WK, Manson JE, Willett WC, et al. Snoring as a risk factor for type II diabetes mellitus: a prospective study. Am J Epidemiol 2002;155:387-93. [Crossref] [PubMed]
- Punjabi NM, Shahar E, Redline S, et al. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol 2004;160:521-30. [Crossref] [PubMed]
- Macey PM, Henderson LA, Macey KE, et al. Brain morphology associated with obstructive sleep apnea. Am J Respir Crit Care Med 2002;166:1382-7. [Crossref] [PubMed]
- Morrell MJ, McRobbie DW, Quest RA, et al. Changes in brain morphology associated with obstructive sleep apnea. Sleep Med 2003;4:451-4. [Crossref] [PubMed]
- Minoguchi K, Yokoe T, Tazaki T, et al. Silent brain infarction and platelet activation in obstructive sleep apnea. Am J Respir Crit Care Med 2007;175:612-7. [Crossref] [PubMed]
- Besedovsky L, Lange T, Haack M. The Sleep-Immune Crosstalk in Health and Disease. Physiol Rev 2019;99:1325-80. [Crossref] [PubMed]
- Ludwig K, Huppertz T, Radsak M, et al. Cellular Immune Dysfunction in Obstructive Sleep Apnea. Front Surg 2022;9:890377. [Crossref] [PubMed]
- Wang Y, Meagher RB, Ambati S, et al. Patients with Obstructive Sleep Apnea Have Altered Levels of Four Cytokines Associated with Cardiovascular and Kidney Disease, but Near Normal Levels with Airways Therapy. Nat Sci Sleep 2021;13:457-66. [Crossref] [PubMed]
- Huppertz T, Horstmann V, Scharnow C, et al. OSA in patients with head and neck cancer is associated with cancer size and oncologic outcome. Eur Arch Otorhinolaryngol 2021;278:2485-91. [Crossref] [PubMed]
- Choi JH, Lee JY, Han KD, et al. Association between obstructive sleep apnoea and breast cancer: The Korean National Health Insurance Service Data 2007-2014. Sci Rep 2019;9:19044. [Crossref] [PubMed]
- Lombardo G, Vena F, Negri P, et al. Worldwide prevalence of malocclusion in the different stages of dentition: A systematic review and meta-analysis. Eur J Paediatr Dent 2020;21:115-22. [PubMed]
- Alhammadi MS, Halboub E, Fayed MS, et al. Global distribution of malocclusion traits: A systematic review. Dental Press J Orthod 2018;23:40.e1-40.e10. [Crossref] [PubMed]
- Miles PG, Vig PS, Weyant RJ, et al. Craniofacial structure and obstructive sleep apnea syndrome--a qualitative analysis and meta-analysis of the literature. Am J Orthod Dentofacial Orthop 1996;109:163-72. [Crossref] [PubMed]
- Banabilh SM, Samsudin AR, Suzina AH, et al. Facial profile shape, malocclusion and palatal morphology in Malay obstructive sleep apnea patients. Angle Orthod 2010;80:37-42. [Crossref] [PubMed]
- Lam B, Ip MS, Tench E, et al. Craniofacial profile in Asian and white subjects with obstructive sleep apnoea. Thorax 2005;60:504-10. [Crossref] [PubMed]
- Johal A, Conaghan C. Maxillary morphology in obstructive sleep apnea: a cephalometric and model study. Angle Orthod 2004;74:648-56. [PubMed]
- Jamieson A, Guilleminault C, Partinen M, et al. Obstructive sleep apneic patients have craniomandibular abnormalities. Sleep 1986;9:469-77. [Crossref] [PubMed]
- Lin L, Zhao T, Qin D, et al. The impact of mouth breathing on dentofacial development: A concise review. Front Public Health 2022;10:929165. [Crossref] [PubMed]
- Santos JF Junior, Abrahão M, Gregório LC, et al. Genioplasty for genioglossus muscle advancement in patients with obstructive sleep apnea-hypopnea syndrome and mandibular retrognathia. Braz J Otorhinolaryngol 2007;73:480-6. [Crossref] [PubMed]
- Behrents RG, Shelgikar AV, Conley RS, et al. Obstructive sleep apnea and orthodontics: An American Association of Orthodontists White Paper. Am J Orthod Dentofacial Orthop 2019;156:13-28.e1. [Crossref] [PubMed]
- Nobile M. THE WHO DEFINITION OF HEALTH: A CRITICAL READING. Med Law 2014;33:33-40. [PubMed]
- Triggs A, Roberson G, Chaudhry K, et al. Screening for obstructive sleep apnea by orthodontists in the United States - A survey study. J Clin Exp Dent 2022;14:e625-32. [Crossref] [PubMed]
- Kunisaki KM, Greer N, Khalil W, et al. Provider Types and Outcomes in Obstructive Sleep Apnea Case Finding and Treatment: A Systematic Review. Ann Intern Med 2018;168:195-202. [Crossref] [PubMed]
- Tarraubella N, Sánchez-de-la-Torre M, Nadal N, et al. Management of obstructive sleep apnoea in a primary care vs sleep unit setting: a randomised controlled trial. Thorax 2018;73:1152-60. [Crossref] [PubMed]
- Epstein LJ, Kristo D, Strollo PJ Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 2009;5:263-76. [Crossref] [PubMed]
- Unal Y, Ozturk DA, Tosun K, et al. Association between obstructive sleep apnea syndrome and waist-to-height ratio. Sleep Breath 2019;23:523-9. [Crossref] [PubMed]
- Ito E, Tsuiki S, Maeda K, et al. Oropharyngeal Crowding Closely Relates to Aggravation of OSA. Chest 2016;150:346-52. [Crossref] [PubMed]
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991;14:540-5. [Crossref] [PubMed]
- Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med 2017;13:479-504. [Crossref] [PubMed]
- Chiu HY, Chen PY, Chuang LP, et al. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP, and Epworth sleepiness scale in detecting obstructive sleep apnea: A bivariate meta-analysis. Sleep Med Rev 2017;36:57-70. [Crossref] [PubMed]
- Kendzerska T, Grewal M, Ryan CM. Utility of Acoustic Pharyngometry for the Diagnosis of Obstructive Sleep Apnea. Ann Am Thorac Soc 2016;13:2019-26. [Crossref] [PubMed]
- Ghegan MD, Angelos PC, Stonebraker AC, et al. Laboratory versus portable sleep studies: a meta-analysis. Laryngoscope 2006;116:859-64. [Crossref] [PubMed]
- Gao X, Li Y, Xu W, et al. Diagnostic accuracy of level IV portable sleep monitors versus polysomnography for pediatric obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med 2021;87:127-37. [Crossref] [PubMed]
- Withers A, Maul J, Rosenheim E, et al. Comparison of home ambulatory type 2 polysomnography with a portable monitoring device and in-laboratory type 1 polysomnography for the diagnosis of obstructive sleep apnea in children. J Clin Sleep Med 2022;18:393-402. [Crossref] [PubMed]
- Cagle JL, Young BD, Shih MC, et al. Portable Sleep Study Device Versus Polysomnography: A Meta-analysis. Otolaryngol Head Neck Surg 2023;168:944-55. [Crossref] [PubMed]
- Grote L, Zou D. Pulse wave analysis during sleep. In: Kryger M, Roth T, Dement WC. editors. Principles and Practice of Sleep Medicine. St. Louis: Elsevier; 2017:1624-1632.e4.
- Conrad D, Rosbe K. Evidence-Based Practice: Pediatric Obstructive Sleep Apnea. In: Rudmik L. editor. Evidence-Based Clinical Practice in Otolaryngology. St. Louis: Elsevier; 2018:83-91.
- Alansari RA. The role of orthodontics in management of obstructive sleep apnea. Saudi Dent J 2022;34:194-201. [Crossref] [PubMed]
- Jacobowitz O, Afifi L, Penzel T, et al. Endorsement of:“treatment of adult obstructive sleep apnea with positive airway pressure: an American academy of Sleep Medicine Clinical Practice Guideline” by World Sleep Society. Sleep Med 2022;89:19-22. [Crossref] [PubMed]
- deBerry-Borowiecki B, Kukwa A, Blanks RH. Cephalometric analysis for diagnosis and treatment of obstructive sleep apnea. Laryngoscope 1988;98:226-34. [Crossref] [PubMed]
- Scannone A, Tosta M, Suarez A, et al. Cephalometric and dental measures as diagnostic tools for the obstructive sleep apnea. J Sleep Disor Treat Care 2017;6:2-7.
- Jayan B, Kadu A. Airway-focused orthodontics. J Indian Orthod Soc 2018;52:23-8. [Crossref]
- Muto T, Yamazaki A, Takeda S. A cephalometric evaluation of the pharyngeal airway space in patients with mandibular retrognathia and prognathia, and normal subjects. Int J Oral Maxillofac Surg 2008;37:228-31. [Crossref] [PubMed]
- Kumar V, Ludlow JB, Mol A, et al. Comparison of conventional and cone beam CT synthesized cephalograms. Dentomaxillofac Radiol 2007;36:263-9. [Crossref] [PubMed]
- Marklund M. Update on Oral Appliance Therapy for OSA. Curr Sleep Med Rep 2017;3:143-51. [Crossref] [PubMed]
- Haviv Y, Bachar G, Aframian DJ, et al. A 2-year mean follow-up of oral appliance therapy for severe obstructive sleep apnea: a cohort study. Oral Dis 2015;21:386-92. [Crossref] [PubMed]
- Gjerde K, Lehmann S, Berge ME, et al. Oral appliance treatment in moderate and severe obstructive sleep apnoea patients non-adherent to CPAP. J Oral Rehabil 2016;43:249-58. [Crossref] [PubMed]
- Sutherland K, Takaya H, Qian J, et al. Oral Appliance Treatment Response and Polysomnographic Phenotypes of Obstructive Sleep Apnea. J Clin Sleep Med 2015;11:861-8. [Crossref] [PubMed]
- Sutherland K, Vanderveken OM, Tsuda H, et al. Oral appliance treatment for obstructive sleep apnea: an update. J Clin Sleep Med 2014;10:215-27. [Crossref] [PubMed]
- Mason M, Welsh EJ, Smith I. Drug therapy for obstructive sleep apnoea in adults. Cochrane Database Syst Rev 2013;CD003002. [Crossref] [PubMed]
- Papandreou C, Schiza SE, Bouloukaki I, et al. Effect of Mediterranean diet versus prudent diet combined with physical activity on OSAS: a randomised trial. Eur Respir J 2012;39:1398-404. [Crossref] [PubMed]
- Johansson K, Hemmingsson E, Harlid R, et al. Longer term effects of very low energy diet on obstructive sleep apnoea in cohort derived from randomised controlled trial: prospective observational follow-up study. BMJ 2011;342:d3017. [Crossref] [PubMed]
- Igelström H, Åsenlöf P, Emtner M, et al. Improvement in obstructive sleep apnea after a tailored behavioural sleep medicine intervention targeting healthy eating and physical activity: a randomised controlled trial. Sleep Breath 2018;22:653-61. [Crossref] [PubMed]
- Igelström H, Emtner M, Lindberg E, et al. Tailored behavioral medicine intervention for enhanced physical activity and healthy eating in patients with obstructive sleep apnea syndrome and overweight. Sleep Breath 2014;18:655-68. [Crossref] [PubMed]
- Aiello KD, Caughey WG, Nelluri B, et al. Effect of exercise training on sleep apnea: A systematic review and meta-analysis. Respir Med 2016;116:85-92. [Crossref] [PubMed]
- Wimms AJ, Kelly JL, Turnbull CD, et al. Continuous positive airway pressure versus standard care for the treatment of people with mild obstructive sleep apnoea (MERGE): a multicentre, randomised controlled trial. Lancet Respir Med 2020;8:349-58. [Crossref] [PubMed]
- Tan B, Tan A, Chan YH, et al. Adherence to Continuous Positive Airway Pressure therapy in Singaporean patients with Obstructive Sleep Apnea. Am J Otolaryngol 2018;39:501-6. [Crossref] [PubMed]
- Demko BG. The Evolution of Oral Appliance Therapy for Snoring and Sleep Apnea: Where Did We Come From, Where Are We, and Where Are We Going? Sleep Med Clin 2018;13:467-87. [Crossref] [PubMed]
- Mickelson SA. Oral Appliances for Snoring and Obstructive Sleep Apnea. Otolaryngol Clin North Am 2020;53:397-407. [Crossref] [PubMed]
- De Vito A, Carrasco Llatas M, Vanni A, et al. European position paper on drug-induced sedation endoscopy (DISE). Sleep Breath 2014;18:453-65. [Crossref] [PubMed]
- Kezirian EJ. Nonresponders to pharyngeal surgery for obstructive sleep apnea: insights from drug-induced sleep endoscopy. Laryngoscope 2011;121:1320-6. [Crossref] [PubMed]
- Barthlen GM, Brown LK, Wiland MR, et al. Comparison of three oral appliances for treatment of severe obstructive sleep apnea syndrome. Sleep Med 2000;1:299-305. [Crossref] [PubMed]
- Poets CF, Koos B, Reinert S, et al. The Tübingen palatal plate approach to Robin sequence: Summary of current evidence. J Craniomaxillofac Surg 2019;47:1699-705. [Crossref] [PubMed]
- Lazard DS, Blumen M, Lévy P, et al. The tongue-retaining device: efficacy and side effects in obstructive sleep apnea syndrome. J Clin Sleep Med 2009;5:431-8. [Crossref] [PubMed]
- Chang ET, Fernandez-Salvador C, Giambo J, et al. Tongue retaining devices for obstructive sleep apnea: A systematic review and meta-analysis. Am J Otolaryngol 2017;38:272-8. [Crossref] [PubMed]
- Chen H, Lowe AA. Updates in oral appliance therapy for snoring and obstructive sleep apnea. Sleep Breath 2013;17:473-86. [Crossref] [PubMed]
- Scherr SC, Dort LC, Almeida FR, et al. Definition of an effective oral appliance for the treatment of obstructive sleep apnea and snoring: a report of the American Academy of Dental Sleep Medicine. J Dent Sleep Med 2014;1:39-50. [Crossref]
- Ferguson KA, Love LL, Ryan CF. Effect of mandibular and tongue protrusion on upper airway size during wakefulness. Am J Respir Crit Care Med 1997;155:1748-54. [Crossref] [PubMed]
- Bartolucci ML, Bortolotti F, Raffaelli E, et al. The effectiveness of different mandibular advancement amounts in OSA patients: a systematic review and meta-regression analysis. Sleep Breath 2016;20:911-9. [Crossref] [PubMed]
- Manetta IP, Ettlin D, Sanz PM, et al. Mandibular advancement devices in obstructive sleep apnea: an updated review. Sleep Sci 2022;15:398-405. [Crossref] [PubMed]
- Basyuni S, Barabas M, Quinnell T. An update on mandibular advancement devices for the treatment of obstructive sleep apnoea hypopnoea syndrome. J Thorac Dis 2018;10:S48-56. [Crossref] [PubMed]
- Quinnell TG, Bennett M, Jordan J, et al. A crossover randomised controlled trial of oral mandibular advancement devices for obstructive sleep apnoea-hypopnoea (TOMADO). Thorax 2014;69:938-45. [Crossref] [PubMed]
- Dieltjens M, Vanderveken O. Oral Appliances in Obstructive Sleep Apnea. Healthcare (Basel) 2019;7:141. [Crossref] [PubMed]
- Isacsson G, Nohlert E, Fransson AMC, et al. Use of bibloc and monobloc oral appliances in obstructive sleep apnoea: a multicentre, randomized, blinded, parallel-group equivalence trial. Eur J Orthod 2019;41:80-8. [Crossref] [PubMed]
- Ishiyama H, Hasebe D, Sato K, et al. The Efficacy of Device Designs (Mono-block or Bi-block) in Oral Appliance Therapy for Obstructive Sleep Apnea Patients: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health 2019;16:3182. [Crossref] [PubMed]
- Aziz R, Somaiah S, Kalha AS, et al. Comparative assessment of changes in pharyngeal airway space in cases of obstructive sleep apnoea with a customized mandibular repositioning appliance - a clinical study. Sleep Sci 2021;14:16-24. [Crossref] [PubMed]
- Murphy M, Remmers J, Mosca E. 0751 The use of a Digitally Milled Oral Appliance in the Treatment of Severe Obstructive Sleep Apnea. Sleep 2022;45:A327-8. [Crossref]
- Schütz TC, Cunha TC, Moura-Guimaraes T, et al. Comparison of the effects of continuous positive airway pressure, oral appliance and exercise training in obstructive sleep apnea syndrome. Clinics (Sao Paulo) 2013;68:1168-74. [Crossref] [PubMed]
- Doff MH, Hoekema A, Wijkstra PJ, et al. Oral appliance versus continuous positive airway pressure in obstructive sleep apnea syndrome: a 2-year follow-up. Sleep 2013;36:1289-96. [Crossref] [PubMed]
- Ferguson KA, Ono T, Lowe AA, et al. A randomized crossover study of an oral appliance vs nasal-continuous positive airway pressure in the treatment of mild-moderate obstructive sleep apnea. Chest 1996;109:1269-75. [Crossref] [PubMed]
- Nikolopoulou M, Byraki A, Ahlberg J, et al. Oral appliance therapy versus nasal continuous positive airway pressure in obstructive sleep apnoea syndrome: a randomised, placebo-controlled trial on self-reported symptoms of common sleep disorders and sleep-related problems. J Oral Rehabil 2017;44:452-60. [Crossref] [PubMed]
- Dort L, Brant R. A randomized, controlled, crossover study of a noncustomized tongue retaining device for sleep disordered breathing. Sleep Breath 2008;12:369-73. [Crossref] [PubMed]
- Ferguson KA, Cartwright R, Rogers R, et al. Oral appliances for snoring and obstructive sleep apnea: a review. Sleep 2006;29:244-62. [Crossref] [PubMed]
- Li P, Ning XH, Lin H, et al. Continuous positive airway pressure versus mandibular advancement device in the treatment of obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med 2020;72:5-11. [Crossref] [PubMed]
- Rangarajan H, Padmanabhan S, Ranganathan S, et al. Impact of oral appliance therapy on quality of life (QoL) in patients with obstructive sleep apnea - a systematic review and meta-analysis. Sleep Breath 2022;26:983-96. [Crossref] [PubMed]
- Ilea A, Timuș D, Höpken J, et al. Oral appliance therapy in obstructive sleep apnea and snoring - systematic review and new directions of development. Cranio 2021;39:472-83. [Crossref] [PubMed]
- Kim HY, Jo JH, Chung JW, et al. The multisystemic effects of oral appliance therapy for obstructive sleep apnea: A narrative review. Medicine (Baltimore) 2022;101:e29400. [Crossref] [PubMed]
- Yoshida K. Effect on blood pressure of oral appliance therapy for sleep apnea syndrome. Int J Prosthodont 2006;19:61-6. [PubMed]
- Trzepizur W, Gagnadoux F, Abraham P, et al. Microvascular endothelial function in obstructive sleep apnea: Impact of continuous positive airway pressure and mandibular advancement. Sleep Med 2009;10:746-52. [Crossref] [PubMed]
- Galic T, Bozic J, Ivkovic N, et al. Effects of mandibular advancement device treatment on arterial stiffness and glucose metabolism in patients with mild to moderate obstructive sleep apnea: a prospective 1 year study. Sleep Breath 2016;20:69-77. [Crossref] [PubMed]
- Marklund M, Franklin KA. Long-term effects of mandibular repositioning appliances on symptoms of sleep apnoea. J Sleep Res 2007;16:414-20. [Crossref] [PubMed]
- McGown AD, Makker HK, Battagel JM, et al. Long-term use of mandibular advancement splints for snoring and obstructive sleep apnoea: a questionnaire survey. Eur Respir J 2001;17:462-6. [Crossref] [PubMed]
- Marklund M. Predictors of long-term orthodontic side effects from mandibular advancement devices in patients with snoring and obstructive sleep apnea. Am J Orthod Dentofacial Orthop 2006;129:214-21. [Crossref] [PubMed]
- Almeida FR, Lowe AA, Sung JO, et al. Long-term sequellae of oral appliance therapy in obstructive sleep apnea patients: Part 1. Cephalometric analysis. Am J Orthod Dentofacial Orthop 2006;129:195-204. [Crossref] [PubMed]
- Almeida FR, Lowe AA, Otsuka R, et al. Long-term sequellae of oral appliance therapy in obstructive sleep apnea patients: Part 2. Study-model analysis. Am J Orthod Dentofacial Orthop 2006;129:205-13. [Crossref] [PubMed]
- Carvalho B, Hsia J, Capasso R. Surgical therapy of obstructive sleep apnea: a review. Neurotherapeutics 2012;9:710-6. [Crossref] [PubMed]
- Koutsourelakis I, Georgoulopoulos G, Perraki E, et al. Randomised trial of nasal surgery for fixed nasal obstruction in obstructive sleep apnoea. Eur Respir J 2008;31:110-7. [Crossref] [PubMed]
- Smith DF, Cohen AP, Ishman SL. Surgical management of OSA in adults. Chest 2015;147:1681-90. [Crossref] [PubMed]
- Mehra P, Wolford LM. Surgical management of obstructive sleep apnea. Proc (Bayl Univ Med Cent) 2000;13:338-42. [Crossref] [PubMed]
- Holty JE, Guilleminault C. Surgical options for the treatment of obstructive sleep apnea. Med Clin North Am 2010;94:479-515. [Crossref] [PubMed]
- Kezirian EJ, Goldberg AN. Hypopharyngeal surgery in obstructive sleep apnea: an evidence-based medicine review. Arch Otolaryngol Head Neck Surg 2006;132:206-13. [Crossref] [PubMed]
- Costello BJ, Posnick JC. The role of maxillofacial osteotomies in the treatment of obstructive sleep apnea. Curr Opin Otolaryngol Head Neck Surg 2003;11:267-74. [Crossref] [PubMed]
- Wolford LM, Chemello PD, Hilliard F. Occlusal plane alteration in orthognathic surgery--Part I: Effects on function and esthetics. Am J Orthod Dentofacial Orthop 1994;106:304-16. [Crossref] [PubMed]
- Wolford LM, Chemello PD, Hilliard FW. Occlusal plane alteration in orthognathic surgery. J Oral Maxillofac Surg 1993;51:730-40; discussion 740-1. [Crossref] [PubMed]
- Vicini C, Dallan I, Campanini A, et al. Surgery vs ventilation in adult severe obstructive sleep apnea syndrome. Am J Otolaryngol 2010;31:14-20. [Crossref] [PubMed]
- de Oliveira CB, Ayub P, Ledra IM, et al. Microimplant assisted rapid palatal expansion vs surgically assisted rapid palatal expansion for maxillary transverse discrepancy treatment. Am J Orthod Dentofacial Orthop 2021;159:733-42. [Crossref] [PubMed]
- Vinha PP, Eckeli AL, Faria AC, et al. Effects of surgically assisted rapid maxillary expansion on obstructive sleep apnea and daytime sleepiness. Sleep Breath 2016;20:501-8. [Crossref] [PubMed]
- Oliven A. Treating obstructive sleep apnea with hypoglossal nerve stimulation. Curr Opin Pulm Med 2011;17:419-24. [Crossref] [PubMed]
- Kezirian EJ, Goding GS Jr, Malhotra A, et al. Hypoglossal nerve stimulation improves obstructive sleep apnea: 12-month outcomes. J Sleep Res 2014;23:77-83. [Crossref] [PubMed]
- Gulotta G, Iannella G, Vicini C, et al. Risk Factors for Obstructive Sleep Apnea Syndrome in Children: State of the Art. Int J Environ Res Public Health 2019;16:3235. [Crossref] [PubMed]
- Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest 2014;146:1387-94. [Crossref] [PubMed]
- Rosen CL, Wang R, Taylor HG, et al. Utility of symptoms to predict treatment outcomes in obstructive sleep apnea syndrome. Pediatrics 2015;135:e662-71. [Crossref] [PubMed]
- Bitners AC, Arens R. Evaluation and Management of Children with Obstructive Sleep Apnea Syndrome. Lung 2020;198:257-70. [Crossref] [PubMed]
- Brietzke SE, Gallagher D. The effectiveness of tonsillectomy and adenoidectomy in the treatment of pediatric obstructive sleep apnea/hypopnea syndrome: a meta-analysis. Otolaryngol Head Neck Surg 2006;134:979-84. [Crossref] [PubMed]
- Yanyan M, Min Y, Xuemei G. Mandibular advancement appliances for the treatment of obstructive sleep apnea in children: a systematic review and meta-analysis. Sleep Med 2019;60:145-51. [Crossref] [PubMed]
- Bucci R, Rongo R, Zunino B, et al. Effect of orthopedic and functional orthodontic treatment in children with obstructive sleep apnea: A systematic review and meta-analysis. Sleep Med Rev 2023;67:101730. [Crossref] [PubMed]
- Camacho M, Chang ET, Song SA, et al. Rapid maxillary expansion for pediatric obstructive sleep apnea: A systematic review and meta-analysis. Laryngoscope 2017;127:1712-9. [Crossref] [PubMed]
- Barton JJ. High-pull headgear versus cervical traction: a cephalometric comparison. Am J Orthod 1972;62:517-29. [Crossref] [PubMed]
- Shavakhi M, Mohamadian F, Zarif Najafi H. The effects of the headgear therapy on the airway dimensions in patients with class II malocclusion: A systematic review. Dent Med Probl 2019;56:191-6. [Crossref] [PubMed]
Cite this article as: Kalha AS, Raju S. Airway-centric orthodontics: a review on oral appliance therapy as a simplified solution to obstructive sleep apnea. Front Oral Maxillofac Med 2024;6:25.