A unique case report of myelodysplastic syndrome with oral manifestations in middle-aged adult patient
Highlight box
Key findings
• A 34-year-old female patient diagnosed with myelodysplastic syndrome (MDS) presented with spontaneous gingival bleeding and gingival hyperplasia and later progressed to necrosis.
What is known and what is new?
• Gingival hyperplasia with necrosis is a known complication of MDS, but it is rare. Oral manifestations are seen in 15–85% of patients with ulceration, bleeding, ecchymosis, and increased susceptibility to viral, fungal, and bacterial infections
• This case report adds to the existing literature by providing a detailed description of the clinical presentation, diagnosis, and management of this condition in a young patient.
What is the implication, and what should change now?
• Main objective is to eliminate potential source of odontogenic and non-odontogenic infection by mechanical debridement and irrigation to control plaque and microbial load.
Introduction
The myelodysplastic syndrome (MDS) describes the rare spectrum of hematological stem cell disorder (1). It is a clonal hematopoietic disorder with ineffective hematopoiesis leading to persistent pancytopenia and defective morphological blood elements (1,2). Older adults are affected, with an incidence rate of 7 per 100,000. The pathophysiology of MDS is unknown. Due to the rampant nature of the syndrome and progression into acute myeloid leukemia (AML), the prognosis is dismal, with a survival rate is only 2.5 years (3). Patients with MDS present with a broad spectrum of oral manifestations. These range from spontaneous gingival bleeding to oral ulcerations and opportunistic infections (4). These manifestations are either due to pancytopenia or the initiation of immunosuppressive therapy. Here we present an unusual case of middle-aged female patient diagnosed with MDS with oral manifestations. This article also highlights the dental considerations for patients with MDS. We present this article in accordance with the CARE reporting checklist (available at https://fomm.amegroups.com/article/view/10.21037/fomm-23-32/rc).
Case presentation
A 34-year-old Asian female patient, experienced palpitations, generalized weakness, and light weariness for six months. No relevant medical, family, or social history was reported. She arrived at the hospital with a fever, chills, rigor, and bleeding from her gums. Skin had a generalized pallor and no petechiae and hematomas were visible. Pancytopenia was confirmed by a hematological investigation (anemia, leukopenia, and thrombocytopenia) (Table 1). Megaloblastic bone marrow with minor dysmyelopoiesis and dysmegakaryopoiesis was observed during a bone marrow aspiration, which raised the possibility of MDS. Additionally, fluorescence in situ hybridization (FISH) analysis revealed monosomy 7 in 10% of the cells, supporting the diagnosis. The patient was advised to start chemotherapy, but she refused treatment. She was educated about the risks and benefits of chemotherapy, as well as the possible consequences of refusing treatment. However, she remained adamant in her decision.
Table 1
| Factors | Data |
|---|---|
| RBC count | 2.3 million cells/cumm |
| Total WBC count | 2,900 cells/cumm |
| Haemoglobin % | 6.9 g/dL |
| Platelet count | 10,000 cells/cumm |
RBC, red blood cells; WBC, white blood cells; cumm, cubic millimetre.
Patient reported on 16 July 2017 with a complaint of spontaneous gingival bleeding. Upon inspection, it was noted that the interdental papilla was blunt and edematous with profuse bleeding, as well as inflamed gingiva with a rolled-out margin. The cervical third of the anterior teeth’s crowns were covered in supragingival calculus (Figure 1). Due to the severe thrombocytopenia, scaling was deferred. She was recalled one week after consulting with the haemato-oncologist and instructed to maintain oral hygiene with 0.2% chlorhexidine mouth wash. After one week, the patient failed to report.
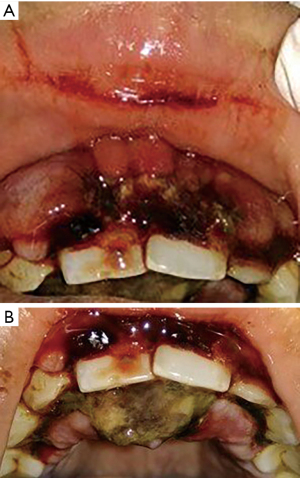
Two months later, she reported a diffuse swelling on the upper lip resulting in incompetent lips and facial asymmetry. Chemotherapy [tablet lenalidomide 5 mg once daily (OD) for 2 months] had commenced at this stage. Diffuse purpuric and petechial spots on arms and legs were noted. On intraoral examination labial surface of the gingiva showed bluish-red diffuse swelling of the gingiva (marginal, attached) and alveolar mucosa from 13 to 23. Gingiva on labial and palatal aspect from 13 to 23 showed areas of necrosis, bleeding, and blood clots. Necrosis of the interdental papilla resulted in the loss of the scalloped architecture of the gingiva. Greyish-yellow pseudomembranous slough covered the areas of necrosis (Figure 2A,2B). A provisional diagnosis of gingival hyperplasia with necrosis secondary to MDS was given. An incisional biopsy was advised once the patient was deemed fit for the procedure. However, patient was lost for follow-up after 2 months of chemotherapy.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent for publication of this case report and accompanying images was not obtained from the patient or the relatives after all possible attempts were made.
Discussion
MDS is defined by the World Health Organization (WHO) as a clonal disease of pluripotent hematopoietic stem cells that is characterized by dysplasia and inefficient hematopoiesis. Pancytopenia, hypercellular bone marrow, organomegaly, dysplasia of blood cells, and megakaryocyte proliferation are some of its distinguishing characteristics. Less than 5% of cases of MDS have bone marrow fibrosis as a co-morbidity (1).
MDS is an uncommon condition that primarily affects persons over the age of 70 years (2). Because AML develops in about 40% of MDS cases, the average survival time is only 2.5 years (4-6). However, our case was 34 years old female. The etiology of MDS is unknown. Most documented cases are idiopathic. Radiotherapy, chemotherapy, treatment for AML and Hodgkin’s disease, and exposure to benzene have all been recognized as predisposing factors. There was no significant history of any predisposing factors in our current case.
According to International Prognostic Scoring System, the three key features that help diagnose are persistent pancytopenia, morphologic dysplasia, and cytogenetic evidence of clonal hematopoiesis. According to WHO classification in 2016 at least 10% of hematopoietic cell lines should display dysplasia (1); 50–60% of patients present with cytogenetic abnormalities as chromosomal loss. The most common cytogenetic abnormalities noted in MDS are deletion of 7q, 5q, and i 17q. In the present case 10 % of cell showed dysplasia and FISH analysis showed deletion of 7q (4-6).
Oral manifestations have been documented by 15–85% of MDS patients (4). These include oral paresthesia, petechiae, hematomas, and burning mouth syndrome in addition to persistent and recurrent mucosal ulcerations (7-9). Patients with MDS frequently experience immunological dysfunction, which increases susceptibility to viral, fungal, and bacterial infections of the oral cavity. Recurrent herpes infection and exfoliative cheilitis are prevalent (6-9).
Another characteristic is the occurrence of hemorrhage in 5% of patients, which can range from petechial hemorrhage to intraoral ecchymosis, hematoma, and spontaneous gingival bleeding (9). Ineffective platelet generation, disorganized megakaryocyte proliferation and maturation, apoptosis, and platelet destruction by auto-antibodies are the causes of the underlying thrombocytopenia that results in bleeding. Spontaneous gingival bleeding and oral ulcerations prevent patients from practicing brushing resulting in the retention of plaque and calculus. This further deteriorates the periodontal status causing periodontal destruction (7,10). The patient in this case experienced gingival hyperplasia, ulceration, necrosis, and spontaneous gingival bleeding. The mainstay of treatment is chemotherapy. Lenalidomide, 5-azacytidine, decitabine, anti-thymocyte globulin, and cyclosporin are some of the medications used. Supportive treatment includes blood transfusion, erythropoietin, deferoxamine, platelet transfusion. Hematopoietic stem cell transplant (HSCT) is the only effective treatment (6).
Comprehensive dental assessment is vital prior to commencement of MDS treatment to minimize the complications associated with the condition and subsequent treatment. This requires a multi-disciplinary team consisting of oral physician, oral surgeon, periodontist, endodontist, dietician, and liaison with haemato-oncologist to deliver effective dental treatment (9). Main objective is to eliminate potential source of odontogenic and non-odontogenic infection. Studies have shown that comprehensive oral and dental care resulted in improved quality of life and reduced morbidity during and after treatment (9). Elad et al. in case base analysis concluded that 1.8 out of 1,000 cases die due to lack of prior dental assessment and oral care (10).
Dental assessment must be performed as early as possible. Traumatic injury to oral mucosa should be anticipated and enameloplasty is performed for sharp tooth/cusp. Fractured restorations and prosthesis are reviewed and eliminated. This helps to reduce traumatic ulcers oral mucosa osteonecrosis. All non-restorable teeth, root remnants, impacted teeth with pericoronitis and periodontally compromised teeth are extracted to eliminate foci of infection. Patient should be encouraged to maintain oral hygiene. Dental plaque is another source of infection in oral cavity. Studies have concluded that dental plaque levels were associated with gingival inflammation and periodontitis. Good plaque control and rigorous oral care lead to decreased colonization of gram negative bacilli and candida species and significant improvement in gingival indices (9,10).
Mechanical debridement and irrigation should be done to control plaque and reduce microbial load. Patients are advised to follow modified bass tooth brushing technique using soft/ultra-soft tooth brush to reduce trauma to gingiva (9,10). In case of severe thrombocytopenia use toothettes or wet cotton soaked in toothpaste to clean the teeth. Pereira et al. recommended patients to discontinue brushing in severe thrombocytopenia (<20×109/L) (11). Any active orthodontic treatment must be discontinued, and fixed orthodontic appliances must be debonded. In case of patient undergoing HSCT, orthodontic treatment is resumed 2 years post bone marrow transplant (12).
MDS patients may experience an acute exacerbation of preexisting chronic pulpal and periodontal diseases during treatment. The treatment at this stage is directed towards symptomatic management which includes pulpotomy, antibiotics, and analgesics. Till the point of disease remission, invasive procedures are deferred. If dental extraction is indicated, a complete blood work up is done. Other considerations include blood transfusions, platelet transfusions, and granulocyte colony-stimulating factor (G-CSF) prior to extraction. Scheduling the appointment is crucial because the extraction is done on the 4th week before the commencement of next chemotherapy cycle. This helps in achieving adequate healing before next cycle (9,10). Patients should receive routine follow-up care while in remission One third of MDS cases can transform into AML (9).
The oral cavity serves as a reflection of one’s overall systemic health. It is crucial for oral physicians to carefully observe and interpret the signs and symptoms exhibited by patients. In some instances, undiagnosed cases of MDS may present with atypical features like gingival hyperplasia, spontaneous gingival bleeding, and intra-oral hematomas. Recognizing the potential connection between these oral manifestations and the MDS is of utmost importance, prompting oral physicians to exercise caution and take proactive measures in evaluating these patients further. Advising comprehensive blood investigations, including a peripheral smear, becomes essential to explore and potentially diagnose such cases. Oral physician plays an important role in identifying these manifestations and referring the patients at the earliest.
This case report provides valuable information about clinical presentation, diagnosis and management of a rare condition MDS with gingival hyperplasia and necrosis. The case report provides a comprehensive overview of the patient’s medical history, clinical presentation, laboratory findings, diagnosis, and management. One of the limitations of this case report is we could not perform scaling and debridement for the patient due to severe pancytopenia.
Conclusions
In conclusion, MDS primarily affects individuals over the age of 70 years, our case involved a 34-year-old female, highlighting the variability in presentation. Dental assessment and comprehensive oral care are crucial before initiating MDS treatment to minimize complications and improve patients’ quality of life. Routine follow-up care is vital due to the potential for MDS to transform into AML. Overall, recognizing and addressing oral manifestations in MDS patients can contribute to timely diagnosis, effective treatment, and improved outcomes. In future advancements in the understanding of molecular genetics, epigenetics, and microRNA patterns may provide insights into the diagnosis, prognosis, and therapies in MDS.
Acknowledgments
We acknowledge the support rendered by Department of Oncology, Yenepoya Medical College and Hospital, Mangalore, Karnataka, India.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://fomm.amegroups.com/article/view/10.21037/fomm-23-32/rc
Peer Review File: Available at https://fomm.amegroups.com/article/view/10.21037/fomm-23-32/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://fomm.amegroups.com/article/view/10.21037/fomm-23-32/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent for publication of this case report and accompanying images was not obtained from the patient or the relatives after all possible attempts were made.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hasserjian RP. Myelodysplastic Syndrome Updated. Pathobiology 2019;86:7-13. [Crossref] [PubMed]
- Flint SR, Sugerman P, Scully C, et al. The myelodysplastic syndromes. Case report and review. Oral Surg Oral Med Oral Pathol 1990;70:579-83. [Crossref] [PubMed]
- Germing U, Kobbe G, Haas R, et al. Myelodysplastic syndromes: diagnosis, prognosis, and treatment. Dtsch Arztebl Int 2013;110:783-90. [PubMed]
- Epstein JB, Priddy RW, Sparling T, et al. Oral manifestations in myelodysplastic syndrome: Review of the literature and report of a case. Oral Surgery, Oral Medicine, Oral Pathology 1986;61:466-70. [Crossref] [PubMed]
- Mangan JK, Chmieliauskaite M, Stoopler ET. An Intraoral Mass Associated With Myelodysplastic Syndrome. JAMA Oncol 2016;2:679-80. [Crossref] [PubMed]
- Mahalakshmi IP, Nagaraj T, Shruthi R, et al. Intraoral ecchymosis in myelodysplastic syndrome with myelofibrosis: A case report. Int J Med Dent Case Rep 2016;3:1-3. [Crossref]
- Prasad SR. Oral manifestations of myeloid neoplasms and acute leukemia- a diagnostic perspective. Hematol Transfus Int J 2018;6:177-80. [Crossref]
- Adeyemo TA, Adeyemo WL, Adediran A, et al. Orofacial manifestation of hematological disorders: hemato-oncologic and immuno-deficiency disorders. Indian J Dent Res 2011;22:688-97. [Crossref] [PubMed]
- Abed H, Alhabshi M, Alkhayal Z, et al. Oral and dental management of people with myelodysplastic syndromes and acute myeloid leukemia: A systematic search and evidence-based clinical guidance. Spec Care Dentist 2019;39:406-20. [Crossref] [PubMed]
- Elad S, Raber-Durlacher JE, Brennan MT, et al. Basic oral care for hematology-oncology patients and hematopoietic stem cell transplantation recipients: a position paper from the joint task force of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) and the European Society for Blood and Marrow Transplantation (EBMT). Support Care Cancer 2015;23:223-36. [Crossref] [PubMed]
- Pereira CM, Gasparetto PF, Coracin FL, et al. Severe gingival bleeding in a myelodysplastic patient: management and outcome. J Periodontol 2004;75:483-6. [Crossref] [PubMed]
- Sheller B, Williams B. Orthodontic management of patients with hematologic malignancies. Am J Orthod Dentofacial Orthop 1996;109:575-80. [Crossref] [PubMed]
Cite this article as: Babu A, Chatra L, Prabhu R. A unique case report of myelodysplastic syndrome with oral manifestations in middle-aged adult patient. Front Oral Maxillofac Med 2025;7:21.