Influence of hypothyroidism on gingival enlargement with periodontitis stage 3 grade B associated with plasma cell gingivitis: a case report
Highlight box
Key findings
• Periodontal disease progression and severity were influenced by hypothyroidism.
What is known and what is new?
• Thyroid dysfunction can impair tissue healing and cause an imbalance in the body’s equilibrium.
• Gingival and periodontal tissue changes associated with thyroid hormone imbalance.
What is the implication, and what should change now?
• Importance of regular professional evaluations, patient education, and persistent educational reinforcement by healthcare practitioners.
Introduction
Background
The main pathologic characteristic of periodontal disease is inflammation. Several risk factors influence an individual’s vulnerability to periodontitis, such as systemic disorders. Periodontal infections affect the progression of several systemic disorders as well as general health. Therefore, it’s a two-way relationship, with periodontal infection and systemic host factors producing broad effects that could hurt the entire body (1).
The most prevalent thyroid condition and most frequent hormone aberration in humans is hypothyroidism. Due to decreased thyroxine (T4), triiodothyronine (T3), and calcitonin production, hypothyroidism can present with a wide range of severity, from asymptomatic individuals to patients experiencing multisystem failure. This can lead to decreased bone metabolism, maturation, and turnover, which can negatively impact bone homeostasis (2).
Rationale and knowledge gap
Evidence points to the possibility that hypothyroidism and periodontitis are related and we hypothesize that this association could be more pronounced in people with more severe periodontitis (2,3).
The findings of Feitosa et al. (2009), who assessed an experimental model of ligature-induced periodontitis in rats with and without thyroid dysfunction, showed that hypothyroidism substantially increased the number of tartrate-resistant acid phosphatase (TRAP)-positive cells on the linear surface of the bone crest and the amount of bone loss due to ligature-induced periodontitis at the ligated locations. Furthermore, no statistically significant variations were seen for the bone quality or the quantity of TRAP-positive cells in the interradicular bone region of ligated teeth between the groups. These results may provide the greatest evidence in favor of that conclusion (3).
In contrast to healthy individuals, hypothyroid patients had greater pocket depths and clinical attachment losses, according to the research by Rahangdale and Galgali (2018) and Yerke et al. (2019), which also showed a connection between periodontitis and hypothyroidism (4,5).
Objective
There is, however, a paucity of information on the connection between periodontal disease and thyroid hormone deficiency. The case report describes a hypothyroidism influence on periodontal destruction. The patient had plasma cell gingivitis, gingival enlargement, and stage 3 grade B periodontitis which was successfully treated and the disease was controlled with surgical periodontal treatment. We present this article in accordance with the CARE reporting checklist (available at https://fomm.amegroups.com/article/view/10.21037/fomm-24-1/rc).
Case presentation
A 35-year-old female patient visited the Periodontics department in October 2023, primarily complaining of swollen, bleeding gums that became enlarged during the previous 6 months.
The patient had a thyroidectomy 7 years prior. The patient had been taking thyroxine 25 mg for hypothyroidism for 7 years; however, the prescription was stopped 6 months ago. Ever since she discovered that her gums suddenly showed spontaneous bleeding episodes and that her gums progressively increased in size till they were aesthetically unpleasant.
The patient’s prior dental history showed that they had received oral prophylaxis and extractions in relation to 16 and 36 before 6 months. However, the gingival enlargement and bleeding did not decrease when the local causes were eliminated.
Based on an intraoral examination, the oral hygiene index score indicated a reasonable level of oral cleanliness (Figure 1). There were bead-shaped interdental papillae and widespread erythematous gingiva with rolled-out borders. Figure 1A clearly shows a widespread lack of scalloping with an enlarged interdental papilla that extended facially and obscured the neighboring tooth surfaces.
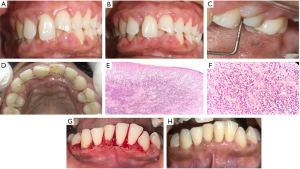
Clinical examination revealed limited local deposits, spontaneous bleeding, a generalized grade II type of inflammatory enlargement with loss of stippling, and the existence of 6–9 mm periodontal pockets (Figure 1B,1C). Splinting was advised on the maxillary anterior teeth (Figure 1D). Hence gingivectomy is indicated and further maintenance is required for a complete reduction in gingival enlargement. Various investigations were advised to the patient, including a complete blood count, periodic thyroid function test, prothrombin time (PT), ultrasonography (USG) abdomen, liver function test (LFT), and blood glucose levels. While her USG report was regular, thyroid function tests revealed decreased T3 (0.6 nmol/L) and elevated thyroid stimulating hormone (TSH =4.6 mU/L). LFT reports and blood glucose levels were also within normal limits.
To assess the systemic thyroid hormone levels, a blood examination was performed because the patient had not been following through on systemic medication. Increased TSH values were more than 4.6 mU/L seen on chemiluminescent immunoassay. Thus, it was seen that the severity of hypothyroidism had increased.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Histopathological examination
A section of soft tissue was taken from the mandibular anterior gingiva and processed for histopathological examination. The hematoxylin and eosin-stained soft tissue sections showed para-keratinized stratified squamous epithelium with superficial cells exhibiting spongiosis and varied rete ridge formation. A focal area of ulceration covered by a fibrinopurulent membrane was observed. The underlying connective tissue was fibro-cellular with irregularly arranged collagen bundles and flat to plump fibroblasts. Mixed inflammatory infiltrate predominantly plasma cells were arranged in the form of sheets beneath the epithelium along with lymphocytes, neutrophils, a few macrophages, and mast cells. Numerous Russell bodies and extravasated red blood cells (RBCs) were evident. Based on the above findings it was diagnosed as plasma cell gingivitis (Figure 1E,1F).
Radiological examination as shown in Figure 2A,2B showed both horizontal and vertical bone loss, with 50–80% of the maxillary anterior and 20–30% of the maxillary posteriors and mandibular anterior having reduced bone. The mandibular anterior teeth showed signs of wear in both horizontal and angular directions, indicating a positive grade II Fremitus test.

Diagnosis
By summarizing the thorough gingival and periodontal examination clinical findings, the case was diagnosed as Periodontitis stage 3 grade B associated with gingival enlargement. Based on histopathological examination, the case was diagnosed as plasma cell gingivitis.
Treatment
TSH levels of 4.5 mU/L were detected in the systemically compromised female patient in the current clinical setting, who also presented with hypothyroidism. Therefore, before any dental procedures were done, she was referred to an endocrinologist. She was therefore provided 25 mg of systemic thyroxine daily. The phase I therapy which included patient education and motivation for oral hygiene along with a complete ultrasonic scaling, was started after the consultation. The abscess was drained with tooth number 11 and kept under amoxicillin 500 mg and Ketorol 10 mg for 3 days. Splinting was done on the maxillary anterior teeth. After that, she was placed on a rigorous maintenance program which refers to supportive periodontal therapy for 8 weeks to assess her adherence to systemic therapy and upkeep of her dental hygiene.
At the 8-week mark, it was seen that while gingival bleeding scores did not significantly improve, there had been additional progress in maintaining dental hygiene. A gingivectomy was done in relation to the lower anterior region as the noticeable enlargement was observed even after thorough non-surgical periodontal therapy (Figure 1G). After 12 weeks, the periodontal charting revealed a significant improvement in the gingival status, with the maxillary anterior teeth being the only teeth still exhibiting periodontal pockets. As a result, a surgical therapy that included pocket reduction in the posterior quadrants was designed. After 6 months, there was a noticeable decrease in the bleeding scores to “0” and an improvement in thyroid hormone levels that were within the normal range of 0.5 to 4.0 mU/L (Figure 1H).
Clinical parameters do not improve much with non-surgical periodontal therapy, which consists of full-mouth scaling and root planing and oral hygiene instructions. However, patients with hypothyroidism and periodontal disease showed improved thyroid function and periodontal health following periodontal surgical procedures.
After that, she was put on a recall program that would check on her compliance with the supportive periodontal therapy and her systemic thyroid hormone levels every 6 weeks. No adverse events were reported by patient during their recall program. The sequence of treatment events is given in a timeline (Figure 3). The patient noticed and learned the link between hypothyroidism and periodontal disease and the impact of treatment lead to improved oral and overall health.
Discussion
Key findings
Although periodontal disease and hypothyroidism are two different medical disorders, there may be a connection between the two based on some data. The body is affected systemically by both disorders, and studies have looked at potential connections between thyroid function and dental health. Here are some things to think about the possible link between periodontal disease and hypothyroidism (6,7).
Due to its immune-modulating effects, hypothyroidism can increase an individual’s susceptibility to infections, especially oral infections. Bacterial infections in the gums are the cause of periodontal disease, and the onset and spread of the condition may be facilitated by an impaired immune system (8).
Increased inflammation is linked to periodontal disease and hypothyroidism. Periodontitis is frequently accompanied by chronic inflammation, and thyroid problems can also cause inflammation in other tissues, including the gums (9).
Thyroid hormones affect the metabolism of connective tissue. The connective tissue that supports teeth deteriorates in periodontal disease. The degree of periodontal disease may be affected by thyroid dysfunction, which may affect the regeneration and repair of connective tissues (10).
A clinical illness state known as hypothyroidism results from the target tissues not having enough thyroid hormone accessible to them. The most accurate markers of thyroid function are serum TSH values. According to the American Thyroid Association, all patients should get a serum TSH measurement at age 35 years and have follow-up every 5 years (11).
In the present case study, gingival enlargement was the most typical issue raised by the patient. The authors described a rare and intriguing case with plasma cell gingivitis, stage 3 grade B periodontitis, associated with hypothyroidism. Such conditions were effectively recognized and treated in a step-by-step manner.
Strengths and limitations
The strengths of this case report are there is histological evidence showing plasma cell gingivitis and with gingivectomy, there is betterment of the condition at 6 months.
The limitations are there is no long-term follow-up and other treatments related to periodontal flap surgeries need to be performed in other regions wherever bone loss is present.
Comparison with similar researches
A study by Yerke et al. (2019) attempted to conjecture how hypothyroidism affected the progression of periodontal disease severity. The study’s authors concluded that hypothyroidism may have an impact on the prevalence of moderate to advanced periodontitis since the data indicated that individuals with hypothyroidism had more teeth with deeper probing depths, suggesting more serious periodontal disease (12).
In their case study, Chowdhary et al. (2017) said that gingival enlargements are the most typical issue that arises in periodontal practice daily. The authors described a rare and intriguing case with plasma cell gingivitis, generalized aggressive periodontitis, associated with hypothyroidism. Such conditions were effectively recognized and treated (13).
Research by Song et al. (2021) demonstrated a potential link between thyroid malfunction and periodontitis. The study’s findings showed a substantial correlation between low TSH levels and a greater risk of periodontitis (14).
In 2023, Khade et al. described the case of a 22-year-old female patient with gingivitis who had a 2-year history of underlying hypothyroidism and was bleeding from her gums at the slightest provocation. She may be mouth breathing due to adenoid enlargement, which can cause obstruction of the airway and obstructive sleep apnea (OSA). Additionally, her maxillary front teeth show more severe gingival inflammatory symptoms than her mandibular anterior teeth (15).
In 2020, Saraswathi et al. studied the impact of nonsurgical periodontal therapy on clinical parameters as well as the serum levels of tumour necrosis factor (TNF)-α and interleukin (IL)-6 in patients with chronic periodontitis who had hypothyroidism or not. It has been discovered that IL-6 and TNF-α are important players in the pathobiology of hypothyroidism and periodontitis. Patients’ periodontal and thyroid conditions are observed to improve as a result of periodontitis treatment (16).
In order to ascertain the correlation between periodontal disease and the related systemic and local risk factors, as well as OSA, Arango Jimenez et al. undertook a study in 2023. The study’s findings indicated that severe OSA was more common in periodontitis patients. The most common systemic findings in patients with OSA and periodontitis were obesity and hypothyroidism (17).
In 2009, Feitosa et al. carried out an animal investigation in rats to assess histologically the impact of thyroid hormones on the quality of alveolar bone that supports teeth and the pace of periodontal bone loss brought on by the implantation of ligatures. The study’s findings indicated that while the alveolar bone, which supports teeth, appears to be less susceptible to changes in hormone levels, lower serum levels of thyroid hormones may exacerbate bone loss associated with periodontitis due to an increase in resorbing cells (3).
Explanations of findings
It is imperative to emphasize that, despite certain evidence suggesting a potential association, more research is necessary to fully comprehend the nature of the interaction between hypothyroidism and periodontal disease. Thus, it serves as a warning to medical practitioners regarding the connection between hypothyroidism and periodontitis, encouraging them to take a multidisciplinary approach (18,19).
Unique periodontal treatments for people with hypothyroidism and periodontal disease include controlling thyroid hormone levels, focusing on anti-inflammatory medicines, and personalizing periodontal care with more frequent scaling and customized treatment regimens. Patients should also receive improved oral hygiene assistance, such as useful manuals and appliances like electric toothbrushes. It’s crucial to manage xerostomia using saliva replacements, stay hydrated, and address any potential nutritional deficits. Lastly, for both conditions to be effectively managed, and a thorough assessment of drugs is essential to prevent interactions (6).
Implications and actions needed
Managing dry mouth, simplifying oral hygiene instructions, and more regular periodontal care are all part of treating a patient with hypothyroidism with periodontal disease (20). In addition, cautious treatment of possible drug interactions and close observation of wound healing and infection risks are necessary. This all-encompassing strategy guarantees efficient treatment for both the periodontal and systemic diseases (21,22).
Conclusions
Certain data points to a possible connection between periodontal disease and hypothyroidism. This correlation may be caused by elements including weakened immune systems, elevated inflammation, decreased salivary flow, and drug side effects. Still, further study is required to completely comprehend the connection. Individuals with hypothyroidism must maintain regular dental care and good oral hygiene habits to stay healthy overall.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://fomm.amegroups.com/article/view/10.21037/fomm-24-1/rc
Peer Review File: Available at https://fomm.amegroups.com/article/view/10.21037/fomm-24-1/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://fomm.amegroups.com/article/view/10.21037/fomm-24-1/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kothiwale S, Panjwani V. Impact of thyroid hormone dysfunction on periodontal disease. Journal of the Scientific Society 2016;43:34-7. [Crossref]
- Ortarzewska M, Nijakowski K, Jankowski J, et al. Periodontal disease in patients with thyroid diseases: A systematic review with meta-analysis. Adv Med Sci 2024;69:289-95. [Crossref] [PubMed]
- Feitosa DS, Marques MR, Casati MZ, et al. The influence of thyroid hormones on periodontitis-related bone loss and tooth-supporting alveolar bone: a histological study in rats. J Periodontal Res 2009;44:472-8. [Crossref] [PubMed]
- Rahangdale SI, Galgali SR. Periodontal status of hypothyroid patients on thyroxine replacement therapy: A comparative cross-sectional study. J Indian Soc Periodontol 2018;22:535-40. [Crossref] [PubMed]
- Yerke L, Levine M, Cohen R. MON-616 Potential Relationship between Hypothyroidism and Periodontal Disease Severity. J Endocr Soc 2019;3:MON-616. [Crossref]
- Inchingolo F, Inchingolo AM, Inchingolo AD, et al. Bidirectional Association between Periodontitis and Thyroid Disease: A Scoping Review. Int J Environ Res Public Health 2024;21:860. [Crossref] [PubMed]
- Williams GR. Actions of thyroid hormones in bone. Endokrynol Pol 2009;60:380-8. [PubMed]
- Fabue LC, Soriano YJ, Pérez MG. Dental management of patients with endocrine disorders. J Clin Exp Dent 2010;2:e196-203.
- Yan B, Ren F, Shang W, et al. Transcriptomic Analysis Reveals Genetic Cross-Talk between Periodontitis and Hypothyroidism. Dis Markers 2022;2022:5736394. [Crossref] [PubMed]
- Baloch Z, Carayon P, Conte-Devolx B, et al. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid 2003;13:3-126. [Crossref] [PubMed]
- Chandna S, Bathla M. Oral manifestations of thyroid disorders and its management. Indian J Endocrinol Metab 2011;15:S113-6. [Crossref] [PubMed]
- Ladenson PW, Singer PA, Ain KB, et al. American Thyroid Association guidelines for detection of thyroid dysfunction. Arch Intern Med 2000;160:1573-5. [Crossref] [PubMed]
- Chowdhary Z, Mohan R, Mehrotra S. An Unusual Association of Gingival Enlargement with Generalized Aggressive Periodontitis Combined with Hypothyroidism and Plasma Cell Gingivitis. Journal of Interdisciplinary Dentistry 2017;7:122-4. [Crossref]
- Song E, Park MJ, Kim JA, et al. Implication of thyroid function in periodontitis: a nationwide population-based study. Sci Rep 2021;11:22127. [Crossref] [PubMed]
- Khade J, Khade AM, Pantawane S, et al. Severe Chronic Gingivitis in Association With Hypothyroidism and Grade 2 Adenoid Hypertrophy: A Case Report. Cureus 2023;15:e49506. [Crossref] [PubMed]
- Saraswathi IR, Sadasivan A, Koshi E, et al. Effect of Nonsurgical Periodontal Therapy on Serum Level of Interleukin-6 and Tumor Necrosis Factor-α in Chronic Periodontitis Patients with and without Hypothyroidism. J Contemp Dent Pract 2020;21:410-5. [Crossref] [PubMed]
- Arango Jimenez N, Morales Vera DZ, Latorre Uriza C, et al. Relationship of obstructive sleep apnea with periodontal condition and its local and systemic risk factors. Clin Oral Investig 2023;27:2823-32. [Crossref] [PubMed]
- Gao Y, Huang D, Liu Y, et al. Periodontitis and thyroid function: A bidirectional Mendelian randomization study. J Periodontal Res 2024;59:491-9. [Crossref] [PubMed]
- Aldulaijan HA, Cohen RE, Stellrecht EM, et al. Relationship between hypothyroidism and periodontitis: A scoping review. Clin Exp Dent Res 2020;6:147-57. [Crossref] [PubMed]
- Aldosary SN, Mirfendereski P, Mupparapu M. A Patient with Hypothyroidism in Need of Periodontal Connective Tissue Graft Surgery. Dent Clin North Am 2023;67:601-3. [Crossref] [PubMed]
- Reddy NR, Kumar PM, Selvi T, et al. Management of Recurrent Post-partum Pregnancy Tumor with Localized Chronic Periodontitis. Int J Prev Med 2014;5:643-7. [PubMed]
- Yuvaraja M, Reddy NR, Kumar PM, et al. Thermoreversible gel for intrapocket delivery of green tea catechin as a local drug delivery system: An original research. J Adv Pharm Technol Res 2016;7:139-43. [Crossref] [PubMed]
Cite this article as: Veeramallu N, Pasupuleti MK, Penmetsa GS, Itha J. Influence of hypothyroidism on gingival enlargement with periodontitis stage 3 grade B associated with plasma cell gingivitis: a case report. Front Oral Maxillofac Med 2025;7:5.