Lingual nerve sensory outcomes of non-grafted microsurgery using platelet rich plasma: retrospective study
Introduction
Trigeminal nerve injuries involving the lingual nerve (LN) are a potential complication of oral and maxillofacial surgical procedures including third molar extractions, local anesthetic injection, treatment of pathology, orthognathic surgery, and maxillofacial trauma. LN injuries affect patients’ quality of life due to their negative impact on speech, taste, swallowing, and possibly development of neuropathic pain. Food and liquid incompetence, speaking and feeling comfortable in social settings may all be adversely affected with LN injuries.
The incidence of reported LN injury secondary to third molar removal was reported as 2% (1). Even though a majority of these injuries are transient and recover spontaneously, the probability of spontaneous recovery has been shown to be 60% at 3 months, 35% at 6 months, and lower than 10% at 9 months or longer (2). Although estimates vary regarding frequency, the literature continues to show that a small number of patients with LN injury sustain permanent neurosensory deficits. The rate of permanent LN injury from third molar surgery has been reported in a number of studies to range from 0.04% to 0.6% (1-7).
Neurotoxicity, as a result of local anesthetic injections, may result in degeneration of axon or myelin cellular structures resulting in nerve injuries. Higher concentration local anesthestics containing 4% anesthetic solutions used in dentistry such as Articaine, (Septodont, Lancaster, PA) are more highly associated with the development of hypoesthesia than those of lower concentrations (7). Pogrel estimated the incidence of permanent nerve injury to occur in 1:160,571 inferior alveolar nerve blocks (8). Orthognathic surgery also has been described to result in trigeminal nerve injuries including the lingual nerve. Most orthognathic related injuries are reported to affect the IAN resulting from mandibular osteotomies ranging from 9% to 84.6% (9). Shawkey’s meta-analysis reported an incidence ranging between 0.3% and 18% with a pooled incidence of 0.7% of permanent lingual nerve damage from orthognathic surgery (10). Restoration of taste after LN injuries has been shown unreliable with improvements reported at approximately 35% (11). Speech can also be affected by LN injuries which has been demonstrated through acoustic analysis to cause the distortion of vowel production that could be perceptually detectable. Lingual nerve impairment has the potential to change speech production as well (12).
Platelet rich plasma (PRP) has been studied since the 1990s and it is known that platelets are capable of secreting growth factors including: platelet-derived growth factor (PDGF), transforming growth factor (TGF), platelet factor interleukin (IL), platelet-derived angiogenesis factor (PDAF), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), insulin-like growth factor (IGF) and fibronectin. Marx postulated that effects of PRP on bone regeneration were due to increased angiogenesis from growth factors secreted from platelets (13). PDGF and IGF-1 play important roles in mitogenic and migration-inducing effects of platelet-rich plasma on human Schwann cells (14). PRP treatment not only showed a significant increase in the number of myelinated axons compared with the control, but also reduced the apoptotic index in a crushed nerve (15). The use of PRP with facial nerve neurorrhaphy has been evaluated and demonstrated an increase in myelination of fibers in comparison without the use of PRP in a rat model. Neurons and Schwann cells express PDGF and PDGF-[beta] receptors which were evaluated qualitatively. The groups that underwent suture repair with PRP appeared to demonstrate increased myelination in comparison with those that did not (16). The procedure of the microsurgical repair has been constantly improving with the innovation of biologic materials. Due to these growth factors, there should be a potential for enhanced nerve repair leading to improved and or accelerated neurosensory outcomes.
Microsurgical repair of the injured LN remains the most effective method of restoring sensation in those patients in whom significant neurosensory deficits has failed to resolve spontaneously. Some of the indications for exploratory LN microsurgery including failure for any spontaneous recovery after 3 months, development of pain or worsening of symptoms and sensory deficit intolerable to the patient. The outcomes of LN microsurgical repair have been described in multiple studies (17-25). No previous studies have specifically investigated the benefits of using PRP in the primary surgical repair of LN injuries. The purpose of the present study was to compare neurosensory outcomes in primary surgical repair of LN patients treated with PRP with those that did not have PRP to determine any difference in FSR as defined by the Medical Research Council Scale (MRCS).
We present the following article in accordance with the STROBE reporting checklist (available at https://fomm.amegroups.org/article/view/10.21037/fomm-21-33/rc).
Methods
After obtaining approval from the biomedical and health sciences institutional review board (reference number CR00003274) of Rutgers University (Newark, NJ), the PI (V.B.Z) operative log was used to find cases of LN repair performed from July 1st, 2015 through February 1st, 2019 without the use of nerve grafts. Patient surgical information was obtained using a retrospective cohort study design by reviewing hospital records and office charts for those patients who underwent exploratory lingual nerve procedures with exception of nerve grafts with or without the use of PRP were included in the study. Patients with bilateral LN repair had each side evaluated separately, so the total number of nerves treated was used in the statistical analysis. Patients who did not present for follow-up for at least 3 months postoperatively were excluded.
Approximately 50 cc of blood was collected in the operating room by the anesthesia team and then was given to the representative of the contracted service for production of PRP. Three services utilized at our institution included American Red Cross, New York Blood Center and Biomet. An average of 5 cc of PRP was obtained from the 50 cc of blood harvested from the patient.
All LN injuries were repaired using standard microsurgical techniques, including the use of a conduit and fibrin glue to secure the conduit without need for additional sutures. The surgical procedures performed were external or internal neurolysis, neuroma excision if applicable, and direct primary neurorrhaphy. This was followed by entubulation with a nerve conduit Axoguard® (Axogen Inc., Alachua, FL), or Neuragen® (IntegraLife, Princeton, NJ) around the circumference of the nerve. The conduit was applied to the nerve repair site and secured with fibrin glue before applying PRP to the nerve and wound bed. 3 cc of PRP was sprayed into a sterile cup and allowed to congeal. The PRP gel was then laid on top of the nerve repair after the fibrin glue was applied. The PRP gel was enclosed in the wound once the flaps were reduced and secured with multiple chromic gut and vicryl sutures. The clinical intraoperative findings of the LN during exploration under magnification determined which surgical procedure(s) was performed.
The PI performed all clinical neurosensory testing preoperatively and at each subsequent follow-up appointment. Subjective neurosensory recovery was determined by standard neurosensory testing including responses to hot, cold, wisp, brush, and pinprick, and objective recovery was determined by testing 2-point discrimination and fine touch threshold with von Frey filaments. The objective findings were correlated to an MRCS score, with grades S3, S3+, and S4 indicating FSR. The subjective measurements of hot, cold, wisp, brush, vibration, and pinprick were converted into a 6-point system (0, no response; 5, normal response; 6, a hyper-response) for statistical analysis. All patient reports of ‘‘decreased’’ were set at 2. The objective measurement dataset were from von Frey filaments and 2-point discrimination. The von Frey filaments size was used as the numeric for comparing the von Frey fiber dataset, and the caliper measurement of 2-point discrimination was used for the 2-point discrimination dataset. However, for calculation, 20 mm was used to substitute for all measurements recorded as larger than 20 mm, making an accurate comparison with the preoperative condition possible.
For analysis and conversion to a numeric system, an FSR of S4 was made equivalent to 5, S3+ to 4, S3 to 3, S2+ to 2 and S2 to 1. The variables were compared using non-parametric tests (Mann Whitney U test), and descriptive statistics for the study variables were computed using IBM SPSS Statistics Version 26 software. The null hypothesis was set as no difference between the application of PRP and the non-use of PRP. Statistical significance was set at a P value equal to 0.05.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Rutgers Institutional Review Board, Study ID: Pro2019000791 and individual consent for this retrospective analysis was waived.
Results
Forty-seven patients were identified who had received surgical treatment for LN injury with or without use of PRP. Of these 47 patients, 7 patients did not present for adequate follow-up for at least 3 months and 14 of the 47 had allogeneic nerve grafts utilized, thus were excluded from the study. 28 patients remained who were included in the present study including 14 patients in each the PRP and non-PRP groups. All LN injuries examined in the present study were the result of third molar extractions.
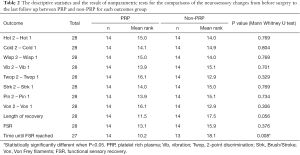
Patients’ ages ranged from 15 to 52 years (mean, 26.6 yr); 36 patients were female (76.5%) and 11 were male (23.5%). The final dataset showed a similar distribution, with 21 female patients (75%) and 7 male patients (25%). 4 male and 10 female patients had PRP included with their treatment. Time from injury to surgery ranged from 2 to 16 months [mean, 4.91 months; standard deviation (SD), 2.42 months]. There was no meaningful difference in time from injury to surgery between the PRP and non-PRP groups. For those patients included in this study, 17 were found to have neurotmesis and 11 with axonotmesis. More patients in the neurotmesis group had PRP (57%) than in the axonotmesis group (39%) as seen in Table 1, but no difference in outcome was noted. As detailed in Table 2, the comparison of pre and post-operative neurosensory changes. The preoperative von Frey fiber test had a mean of 6.3 (SD, 0.65), which improved for the 2 groups postoperatively (PRP group: mean, 2.43; SD, 1.09; non-PRP group: mean, 2.0; SD, 0) but, showed a lack of statistical significance between the results (P value: 0.274). With 2-point discrimination, the preoperative mean was (greater than) 19.43 mm (SD, 1.91 mm). Postoperatively, the PRP group had a mean of 10.93 mm (SD, 3.43 mm), whereas the non-PRP group had a mean of 9.79 mm (SD, 3.29 mm). This also failed to show statistical significance between outcomes (P value 0.326).

Full table
Full table
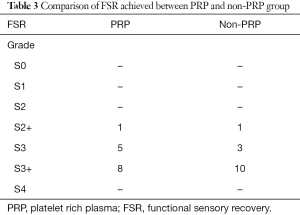
The total FSR per group is listed in Table 3. Two of the twenty-eight patients MRCS score was S2+ thus did not achieve FSR. The mean FSR for the PRP group was 3.43 (SD 0.646) and for the non-PRP group was 3.64 (non-PRP with SD 0.633) or equivalent to S3+. There were no statistically relevant differences in FSR outcomes between the groups (P value 0.384). Table 4 shows the comparison of follow up between the PRP and non-PRP groups in regards to FSR and time until S3 reached. Follow-up time was from 3 to 18 months (mean, 9.32 months; SD, 3.67 months). The time until achieving the FSR was 5.5 months for the PRP group and 9.5 for the non-PRP group.
Full table

Full table
Non-parametric tests (Mann Whitney U test) were shown that there was not a significantly different change in neurosensory function from prior to surgery to the last follow up between PRP and NON-PRP groups for all outcomes (all P values >0.05) except time until FSR reached. There was a statistical significant difference in time until FSR reached between the PRP and non-PRP groups (P=0.008). PRP group had significantly less time until FSR reached than non-PRP group.
Discussion
Microsurgical peripheral nerve repair has been shown to be effective for neurosensory recovery, but as techniques have become standardized, biologic products have been the focus of most recent research. Biologic products including allogeneic nerve grafts, blood products such as PRP and adipose derived stem cells are now the focus of study. Platelet-rich plasma is a minimally invasive autogenous source of growth factors that has been shown to aid in soft tissue and bone healing. Although platelet counts average range between 150,000–400,000 per microliter, proof of bone and soft tissue healing enhancement have been shown using a platelet-rich plasma with platelet counts of 1,000,000 per microliter (26). With specific regards to nerve healing, PRP has been shown to facilitate increased myelination of fibers, decreased apoptosis of crushed nerves, and increased mitogenic and migration-inducing effects on Schwann cells (15,16).
In the present study, neurosensory outcomes of patients treated using topical application of PRP were compared with those patients treated in the same manner without PRP, and FSR determined by an MRCS score of at least S3. Even though the non-PRP group had more patients with FSR of 3+; the von Frey and the 2-point discrimination were within 1 standard deviation of each other. Thus, there is no statistical difference, likely due to the small sample size. Moreover, a statistically significant difference was identified in the length of time until achieving FSR S3. This was evaluated by looking into each patients’ progress notes and identifying once the patient met the criteria for FSR S3. For the PRP group, the average time status post-surgery until patients’ FSR S3 reached was 5.5 months with a standard deviation of 1.4 and the non-PRP group was 9.5 months with a standard deviation of 4.16. These results are statistically significant (P value of 0.008) confirming that PRP aids in the acceleration of nerve regeneration but not a difference in final FSR achieved
As with all studies, one must account for limitations and biases. This was a retrospective study thus selection bias is of concern however given the low prevalence of this injuries and repairs we would not try to exclude more patient but try to include more. To be critical of this study, I would argue an inclusion criteria with a minimum follow up of 3 months was short. Lingual nerve neurosensory outcomes are usually not finalized until 1 to 2 years. However, the result of time until FSR was achieved would have likely not have changed since we were determining how quickly one can achieve FSR.
Conclusions
Although there was no statistical difference in outcomes of FSR, this is likely due to the limited sample size. Moreover, the time to FSR S3 was reduced for the PRP group in comparison to the non-PRP group confirming that PRP aids in the acceleration of healing. Of note, there were no infections or wound dehiscences noted on all of the patients who had lingual nerve repair with PRP. The authors believe that the use of PRP enhanced soft tissue healing with the reduction in wound dehiscence and patient discomfort. Use of PRP should be a consideration in the management of lingual nerve injuries and exploratory microsurgery. We recommend further studies to evaluate effects of PRP on FSR with a larger sample size in addition to evaluate the benefit when used with a nerve graft.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://fomm.amegroups.org/article/view/10.21037/fomm-21-33/rc
Data Sharing Statement: Available at https://fomm.amegroups.org/article/view/10.21037/fomm-21-33/dss
Peer Review File: Available at https://fomm.amegroups.org/article/view/10.21037/fomm-21-33/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://fomm.amegroups.org/article/view/10.21037/fomm-21-33/coif). VBZ serves as an unpaid editorial board member of Frontiers of Oral and Maxillofacial Medicine from Nov 2019 to Oct 2021. VBZ serves as a consultant for Axogen, Alachua, FL. The other authors have no conflict of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Rutgers Institutional Review Board, Study ID: Pro2019000791 and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Valmaseda-Castellón E, Berini-Aytés L, Gay-Escoda C. Lingual nerve damage after third lower molar surgical extraction. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000;90:567-73. [Crossref] [PubMed]
- Bagheri SC, Meyer RA, Khan HA, et al. Retrospective Review of Microsurgical Repair of 222 Lingual Nerve Injuries. J Oral Maxillofac Surg 2010;68:715-23. [Crossref] [PubMed]
- Queral-Godoy E, Figueiredo R, Valmaseda-Castellon E, et al. Frequency and evolution of lingual nerve lesions following lower third molar extraction. J Oral Maxillofac Surg 2006;64:402. [Crossref] [PubMed]
- Blackburn CW, Bramley PA. Lingual nerve damage associated with the removal of lower third molars. Br Dent J 1989;167:103. [Crossref] [PubMed]
- Mason DA. Lingual nerve damage following lower third molar surgery. Int J Oral Maxillofac Surg 1988;17:290. [Crossref] [PubMed]
- Hillerup S, Stoltze K. Lingual nerve injury in third molar surgery. Observations on recovery of sensation with spontaneous healing. Int J Oral Maxillofac Surg 2007;36:884. [Crossref] [PubMed]
- Garisto GA, Gaffen AS, Lawrence HP, et al. Occurrence of paresthesia after dental local anesthetic administration in the United States. J Am Dent Assoc 2010;141:836-44. [Crossref] [PubMed]
- Pogrel MA, Thamby S. Permanent nerve involvement resulting: from inferior alveolar nerve blocks. J Am Dent Assoc 2000;131:901-7. [Crossref] [PubMed]
- Al-Bishri A, Barghash Z, Rosenquist J, et al. Neurosensory disturbance after sagittal split and intraoral vertical ramus osteotomy: as reported in questionnaires and patients’ records. Int J Oral Maxillofac Surg 2005;34:247-251. [Crossref] [PubMed]
- Shawky M, Mosleh M, Jan M, et al. Meta-analysis of the Incidence of Lingual Nerve Deficits After Mandibular Bilateral Sagittal Split Osteotomy. J Craniofac Surg 2016;27:561-4. [Crossref] [PubMed]
- Scrivani SJ, Moses M, Donoff RB, et al. Taste perception after lingual nerve repair. J Oral Maxillofac Surg 2000;58:3-5. [Crossref] [PubMed]
- Niemi M, Laaksonen JP, Vähätalo K, et al. Effects of transitory lingual nerve impairment on speech: An acoustic study of vowel sounds. J Oral Maxillofac Surg 2002;60:647-52. [Crossref] [PubMed]
- Marx RE, Carlson E, Eichstaedt R, et al. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1998;85:638-46. [Crossref] [PubMed]
- Sowa Y, Kishida T, Tomita K, et al. Involvement of PDGF-BB and IGF-1 in Activation of Human Schwann Cells by Platelet-Rich Plasma. Plast Reconstr Surg 2019;144:1025e-1036e. [Crossref] [PubMed]
- Bastami F, Vares P, Khojasteh A. Healing Effects of Platelet-Rich Plasma on Peripheral Nerve Injuries. J Craniofac Surg 2017;28:e49-e57. [Crossref] [PubMed]
- Farrag TY, Lehar M, Verhaegen P, et al. Effect of Platelet Rich Plasma and Fibrin Sealant on Facial Nerve Regeneration in a Rat Model. Laryngoscope 2007;117:157-65. [Crossref] [PubMed]
- Zuniga JR, Chen N, Phillips CL. Chemosensory and somatosensory regeneration after lingual nerve repair in humans. J Oral Maxillofac Surg 1997;55:2. [Crossref] [PubMed]
- Lam NP, Donoff RB, Kaban LB, et al. Patient satisfaction after trigeminal nerve repair. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003;95:538. [Crossref] [PubMed]
- Susarla SM, Lam NP, Donoff RB, et al. A comparison of patient satisfaction and objective assessment of neurosensory function after trigeminal nerve repair. J Oral Maxillofac Surg 2005;63:1138. [Crossref] [PubMed]
- Susarla SM, Kaban LB, Donoff RB, et al. Does early repair of lingual nerve injuries improve functional sensory recovery? J Oral Maxillofac Surg 2007;65:1070. [Crossref] [PubMed]
- Pogrel MA. The results of microneurosurgery of the inferior alveolar and lingual nerve. J Oral Maxillofac Surg 2002;60:485. [Crossref] [PubMed]
- Robinson PP, Loescher AR, Smith KG. A prospective, quantitative study on the clinical outcome of lingual nerve repair. Br J Oral Maxillofac Surg 2000;38:255. [Crossref] [PubMed]
- Robinson PP, Smith KG. A study on the efficacy of late lingual nerve repair. Br J Oral Maxillofac Surg 1996;34:96. [Crossref] [PubMed]
- Rutner TW, Ziccardi VB, Janal MN. Long-term outcome assessment for lingual nerve microsurgery. J Oral Maxillofac Surg 2005;63:1145. [Crossref] [PubMed]
- Renton T. Lingual nerve assessment and repair outcomes. Ann R Australas Coll Dent Surg 2002;16:113. [PubMed]
- Marx RE. Platelet-Rich Plasma (PRP): What Is PRP and What Is Not PRP? Implant Dent 2001;10:225-8. [Crossref] [PubMed]
Cite this article as: Serratelli D, Ziccardi VB, Jiang S. Lingual nerve sensory outcomes of non-grafted microsurgery using platelet rich plasma: retrospective study. Front Oral Maxillofac Med 2021;3:23.